In vivo cytotoxicity of MgO-doped nanobioactive glass particles and their anticorrosive coating on Ti–6Al–4V and SS304 implants for high load-bearing applications
Abstract
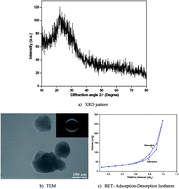
In this study, magnesium-doped nanobioactive glass (NBG) composites (SiO2–CaO–P2O5–MgO) were prepared by simple sol–gel method, which were characterized and coated on Ti alloy (Ti–6Al–4V) and stainless steel (SS304) implants by spin-coating technique. The prepared nanocomposite shows amorphous nature and spherical morphology with particle size of less than 100 nm. The adsorption and desorption isotherms showed the prepared nanocomposites to be in mesoporous range with a specific surface area of 104.1 m2 g−1. The coated implant was found to have a uniform structure without any cracks and pores. Magnesium-doped NBG-coated Ti implants show high corrosion resistance and hardness. In addition, formation of bone-like apatite layer on the coated implant was found to be high in magnesium-doped NBG particles. In addition, in vivo toxicity of the glasses was studied in zebrafish (Danio rerio) embryos, and the results confirmed significant toxicity at higher concentration. Hence, magnesium-doped NBG-coated implant is found to be a potential nanocomposite for high load-bearing applications with better anticorrosive property and long-term stability.

 Please wait while we load your content...
Please wait while we load your content...