Unusual post-translational protein modifications: the benefits of sophistication
Abstract
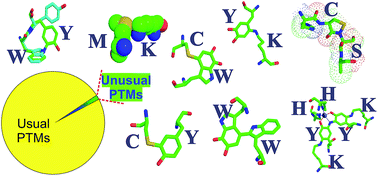
The proteome of an organism represents the work force that is responsible for cellular activities, regulation and survival. Subsequent to synthesis and folding, there is growing evidence that proteins can undergo several novel and previously unknown post-translational modifications (PTMs) that are structurally and functionally significant. Non-disulphide backbone–side chain or side chain–side chain covalent bonds and supplementary modifications that chiefly generate catalytic centres and render proteinaceous enzymes functionally autonomous, are highlighted in this review. Currently known biosynthetic mechanisms derived using modern methodology for the identification of such PTMs are discussed.


 Please wait while we load your content...
Please wait while we load your content...