Performance and emission analysis of a multi cylinder gasoline engine operating at different alcohol–gasoline blends
Abstract
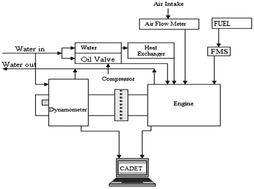
Alcohols are potential renewable alternatives for gasoline because of their bio-based origin. Although ethanol has been successfully implemented in many parts of the world, other alcohols may also be utilized, such as methanol, propanol, and butanol. These alcohols contain much energy and a high octane number. Furthermore, they displace petroleum. Therefore, this study focuses on methanol, ethanol, propanol, and butanol as gasoline fuel alternatives. We conducted tests in a four-cylinder gasoline engine under the wide open throttle condition at varying speeds and results. This engine was fueled with 20% methanol–80% gasoline (M20), 20% ethanol–80% gasoline (E20), 20% propanol–80% gasoline (P20), and 20% butanol–80% gasoline (B20). M20, E20, P20, and B20 displayed brake specific fuel consumptions levels and break thermal efficiencies that were higher than those of gasoline at 7.78%, 5.17%, 4.43%, and 1.95% and 3.6%, 2.15%, 0.7%, and 1.86%, respectively. P20 and B20 showed better torque than E20, but they consumed more fuel. Moreover, the alcohol–gasoline blends generated a higher peak in-cylinder pressure than pure gasoline. As gasoline fuel alternatives, propanol and butanol were more effective than gasoline in engines. In addition, the alcohol–gasoline blends also emitted less carbon monoxide and hydrocarbon than gasoline. However, E20 emitted more nitrogen oxide than the other alcohol–gasoline blends. Thus, propanol and butanol are more effective options than ethanol for a gasoline engine in terms of fuel properties, engine performance, and emissions.

 Please wait while we load your content...
Please wait while we load your content...