Self-assembled hairy ball-like V2O5 nanostructures for lithium ion batteries†
Dong Fanga,
Licheng Lia,
Weilin Xu*a,
Guangzhong Lib,
Zhiping Luoc,
Chaowei Lianga,
Yongsheng Jia,
Jie Xua and
Chuanxi Xionga
aKey Lab of Green Processing and Functional Textiles of New Textile Materials, Ministry of Education, College of Material Science and Engineering, Wuhan Textile University, Wuhan, P. R. China. E-mail: csufangdong@gmail.com
bState Key Laboratory of Porous Metal Material, Northwest Institute for Non-ferrous Metal Research, Xi'an, P. R. China
cDepartment of Chemistry and Physics and Southeastern North Carolina Regional Microanalytical and Imaging Consortium, Fayetteville State University, Fayetteville, USA
First published on 30th May 2014
Abstract
Hairy ball-like nanostructures assembled from nanowires, are synthesized by a hydrothermal method. The reaction time has a significant effect on the morphologies of the products. After calcination at 360 °C, the precursor can be transformed to hairy ball-like V2O5 nanostructures, which present an excellent electrochemical performance for lithium ion batteries.
Lithium-ion batteries (LIBs) are one of the fastest developing electrical energy storage devices nowadays,1–4 and recent developments in nanotechnology and materials science have provided possible resources to satisfy the ever-growing demands for better performance with higher capacities and longer cycle life.5,6 Among the potential cathode materials, V2O5 with a layered structure has been extensively studied in recent years because of its low cost, abundance, ease of synthesis, as well as its high energy efficiency and relatively high theoretical capacity (about 294 mA h g−1 with 2 Li insertion/extraction per unit formula).7–9 However, the performance of V2O5 is significantly limited by a slow rate of lithium diffusion in the lattice and low electronic conductivity.10
Many works have suggested the electrochemical properties of the V2O5 are strongly dependent on their morphology and microstructure.11,12 Various methods, hydrothermal growth,13 sol–gel synthesis,14 electrostatic spray deposition,15 anodic oxidation,16 electrosping,17 atomic layer deposition18 and electrochemical deposition19 etc., have been used to fabricate the V2O5 with various morphologies in different dimensions, including nanorod,20 nanotubes,16 nanobelts,21 nanowire,22,23 nanosheet,13 nanofiber17 and 3D porous hierarchical microspheres7 etc. Beside these morphologies, hairy ball-like morphology of three dimensional (3D) divergent structures with dense nanowires emanating from the center, may possess superior performance as it combines the features of high specific surface and large porosity together. The nanowires can provide active centers with shortened path length for Li+ transport, while the 3D hairy ball-like structure with large porosity may accommodate larger volume expansion during the redox action of the electrodes, which is ideal for the application in LIBs, especially for the alleviation of the poor capacity retention.
In this study, self-assembled hierarchical nanostructures have been successfully prepared via a hydrothermal method to initially synthesize the NH4V4O10 precursor, which is then followed with annealing in air to obtain V2O5. The V2O5 product displays novel hairy ball-like nanostructures composed of nanowires. The morphology and electrochemical properties of the prepared materials are investigated in detail in the following sections.
The XRD pattern of the as-prepared sample is presented in Fig. S1(a) (ESI†) and all of the diffraction peaks can be perfectly indexed as monoclinic ammonium vanadium bronze (NH4V4O10) (PDF#31-0075) with lattice parameters a = 11.71 Å, b = 3.66 Å, c = 9.72 Å and β = 101°. No impurities, such as V2O5 or VO2 can be detected in this pattern, indicating that the pure NH4V4O10 could be obtained using the current synthesis conditions. The chemical compositions of the as-prepared NH4V4O10 are characterized using XPS. The C 1s peak at 284.6 eV is used as a reference binding energy for calibration. A wide survey scan X-ray photoelectron spectrum is shown in Fig. S1(b) (ESI†). A series of peaks from N 1s, V 2p, C 1s and O 1s are clearly observed. The carbon peak of the sample is from surface contamination of CO2.24 Fig. S1(c) (ESI†) shows the enlarged spectrum between 514 eV and 521 eV of the sample. The peak at 516.2 eV with the FWHM of 2.16 eV is attributed to the V2p3/2 that is centered at the V4+ position. The other peak at 517.6 eV with the FWHM of 2.09 eV corresponds to the binding energy of the V2p3/2 electrons for vanadium in the +5 oxidation state.25 The molar ratio of the V4+ to V5+ ions in the as-prepared sample was about 1/3.
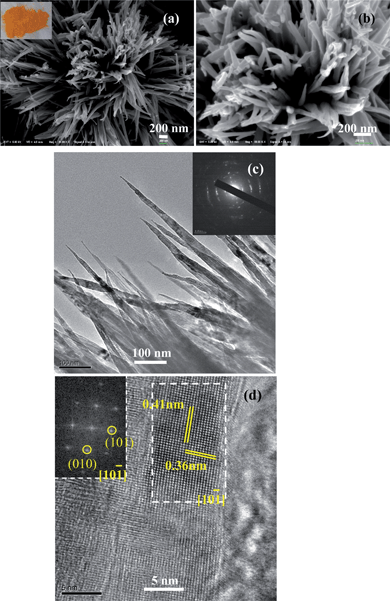
The morphology of the as-prepared dark-blue powder was investigated using SEM (Fig. 1(a)), which reveals uniform hairy ball-like spheres with about 8 μm in diameter. We find that each single hairy ball-like sphere is assembled with nanowires which seem to share a common centre and grow outwards along the radial direction (Fig. 1(b)). The detailed structure and the growth direction of the NH4V4O10 nanowires are further examined by TEM. Fig. 1(c) shows a single ball in a lower magnification, and several nanowires are shown in the inset of Fig. 1(d), which has a diameter of approximately 20 nm with smooth surfaces. The high resolution TEM (HR-TEM) image in Fig. 1(d) is taken from the area marked by rectangle frame in the inset. The nanowire exhibits low crystallinity with limited areas of lattice fringes.
The morphologies of the products at different reaction times were also examined. Fig. S2(a)–(d) (ESI†) show representative SEM images of the samples, which were collected stepwise after 30 min, 1 h, 3 h and 5 h of reaction, and a schematic representation of the morphological evolution process of the hairy ball-like NH4V4O10 is presented in Fig. S2(e).† After 30 min of reaction time, fan-shaped nanowires aggregates were formed (Fig. S2(a)†). In the products in 40 min of reaction time, the nanowires were further assembled to the hair-ball like structure (as-shown in Fig. 1(a)). After 1 h of reaction time (Fig. S2(b)†), the spaces among the nanowires were cross-linking and thin film structures were emerged, and this phenomenon was further evidently presented after 3 h (Fig. S2(c)†) and 5 h (Fig. S2(d)†) of reaction. Besides, the formation of the hairy ball-like structures was also studied at shorter reaction times of 10 or 20 minutes. We found that there were no precipitates when the reaction time was 10 or 20 minutes, and the solution remained dark blue indicating little or no reaction occurred.
Thermal behavior of the precursor NH4V4O10 was investigated by TG and its corresponding DSC analysis. Fig. S3(a) (ESI†) shows the TG curves of the sample in the temperature range of 30–500 °C. It is found that the initial weight loss takes place during 30–187 °C is mainly attributed to the removal of physically adsorbed water. As the temperature rises to 187 °C the weight loss drops slowly with 4.9% and finally it flattens at about 360 °C, which is mainly caused by the decomposition of the solid precursor into V2O5, NH3 and H2O in the presence of oxygen during measurement in air. The decomposition is expressed using the following reaction:
After calcinations at 360 °C in air, the as-prepared NH4V4O10 converts into crystalline V2O5 (Fig. S3(b) (ESI†)). All the diffraction peaks can be indexed to orthorhombic V2O5 phase (JCPDS 89-0612) with lattice parameters a = 11.544 Å, b = 3.571 Å, and c = 4.383 Å. No impurity phase can be detected from the diffraction pattern.
After annealing at 360 °C, the morphological and structural characterizations of yellow V2O5 powder were carried out using both SEM and TEM (HR-TEM). The samples keep their original hair-ball like structure and the size of each nanowires is lager than that of the as-prepared NH4V4O10 nanowires, which is due to the adjacent nanowires reunited into a nanowire during annealing (Fig. 2(a) and (b)). The image of V2O5 nanowires characterized using TEM is shown in Fig. 2(c). The nanowires with a conical structure are consistent with the observed SEM image. An HR-TEM image of V2O5 nanocrystals with lattice fringes is shown in Fig. 2(d), and the corresponding Fast Fourier Transform (FFT) pattern is shown in inset of Fig. 2(d). The interplanar spacings are measured as 0.41 nm and 0.36 nm, corresponding to the spacing of (101) and (010) crystal planes of V2O5, respectively.
The effect of annealing temperature was further researched, according to the SEM images of hairy ball-like V2O5 nanostructures annealed at different temperatures (Fig. 3), it can be found that the adjacent nanowires structure become aggregation to form big sticks after annealing at 400 °C (Fig. 3(a) and (b)). When the annealing temperature is up to 500 °C, the size of big sticks is even larger and the nanowires structure are completely destroyed (Fig. 3(c) and (d)). For comparation, lower resolution SEM images of V2O5 calcined at different temperatures have been provided in Fig. S4 (ESI†). The size of V2O5 spheres annealed at 500 °C is smaller than that annealed at 360 °C or 400 °C. The XRD patterns of V2O5 spheres after annealing at 400 and 500 °C shown in Fig. S5 (ESI†) are similar to that of V2O5-360 °C.
 | ||
| Fig. 3 SEM images of hairy ball-like spheres after annealing at different temperature for 5 h, (a and b) 400 °C; (c and d) 500 °C. | ||
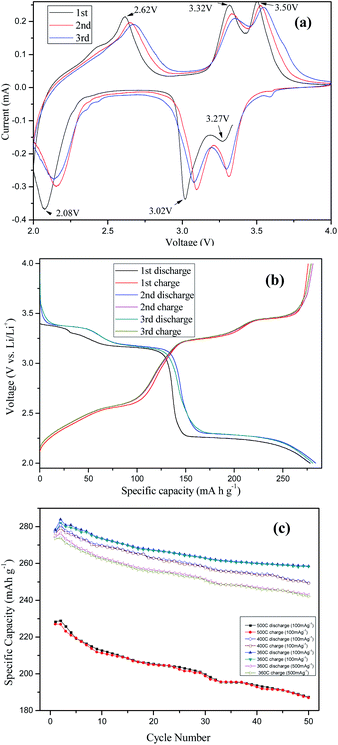
The hair-ball V2O5 nanostructures are evaluated as a cathode material for LIBs in view of their many appealing structural features. Fig. 4(a) shows the first three consecutive cyclic voltammograms (CVs) of the V2O5-360 °C. The insertion of the first Li+ ion into V2O5 follows a two-step process. As a result, the two cathodic peaks at 3.27 and 3.02 V (vs. Li/Li+) correspond to phase changes from a-V2O5 to ε-Li0.5V2O5 and then to δ-LiV2O5, respectively. The third cathodic peak at 2.08 V is attributed to the intercalation of the second Li+ ion, leading to the formation of γ-Li2V2O5.26 Three anodic peaks observed at 2.62, 3.32 and 3.50 V are ascribed to the Li+ ion deintercalation process and the corresponding reverse phase transformations from γ-Li2V2O5 to δ-LiV2O5, ε-Li0.5V2O5, and a-V2O5, respectively.27 In the following cycles, the peaks positions are different slightly. Fig. 4(b) shows the first three cycles of discharge (Li insertion) and charge (Li extraction) curves of the V2O5 electrode in the voltage window of 2.0–4.0 V at a current density of 100 mA g−1, consistent with the CV scans. Multiple voltage plateaus due to different redox reactions associated with Li insertion are clearly observed in the discharge curves. Three plateaus are well observed at 3.3, 3.1 and 2.2 V on the discharge curves, which indicates the multi-step Li+ ion intercalation process. Three corresponding plateaus related to the Li+ ion deintercalation process are also observed on the charge curves. The discharge–charge voltage profiles are in good agreement with the CV results. The sample delivers a high initial discharge capacity of 278.3 mA h g−1 and the value slightly increases to 283.9 mA h g−1 in the second cycle probably due to improved electrolyte penetration. This value realizes 96.6% of the theoretical capacity for two Li+ ions intercalation (294 mA h g−1). The value decreases to 280.8 mA h g−1 in the third cycles and the generally overlapping profiles indicate the good reversibility of the electrochemical processes upon cycling.
The cycling performance for the V2O5-360 °C, V2O5-400 °C and V2O5-500 °C electrodes at 100 mA g−1 or 500 mA g−1 were presented in Fig. 4(c). After 50 cycles, specific discharge capacities of 258.5, 249.3 and 187.4 mA h g−1 can be retained for V2O5-360 °C, V2O5-400 °C and V2O5-500 °C electrodes at 100 mA g−1, respectively. The coulombic efficiency (as shown in Fig. S6 (ESI†)) of the V2O5-360 °C electrode is close to 100%, suggesting the good reversibility for the Li+ ion insertion/deinsertion processes. The Brunauer–Emmett–Teller (BET) specific surface area for V2O5-360 °C has been estimated to be 14.72 m2 g−1, which is larger than that for V2O5-500 °C (5.01 m2 g−1). High surface area could lead to more side reaction, including the decomposition of the electrolyte, impacting on the cycling stability of the materials, further which will lead to a large irreversible capacity in the first cycle.28 While in Fig. 4(b) and (c), the large irreversible capacity was not found during the first several charge–discharge cycles. The side reaction for V2O5 nanowires electrode was not an obvious feature, which is coincident with the results of uniform V2O5 nanosheet29 and V2O5 nanowire/grapheme23 with excellent lithium storage properties. In other hand, the relatively large surface area of the V2O5 nanowires ensures short diffusion distances of Li ions and therefore holds the promise for enhanced Li storage performance.30–32 The V2O5-360 °C is also cycled at a much higher current density of 500 mA g−1 and a specific discharge capacity of 274.3 mA h g−1 can be obtained in the first cycle and 242.9 mA h g−1 is obtained after 50 cycles.
Conclusions
We implemented a hydrothermal route to prepare NH4V4O10 hairy ball-like nanostructures that are assembled from smaller building units of nanowires. In this synthetic route, the morphologies of the products were evolution along with the reaction times, that is, nanowires were assembled to hairy ball-like structure firstly, and then thin film structures among the spaces of the nanowires were emerged and grown. Based on this solid precursor, we have successfully prepared hairy ball-like V2O5 nanowires via direct thermal decomposition in air at 360 °C. As the anode material for lithium ion batteries, the hairy ball-like V2O5-360 °C electrode exhibited high lithium storage capacity, good cycling stability, and superior rate capability. The excellent electrochemical performance is attributed to the large specific surface area of the hairy ball-like V2O5 structure, which is well accessible for electrolyte diffusion and intercalation of Li+ ions into the active phases.Acknowledgements
This work was supported by the Natural Science Foundation of China (no. 51201117, 51104121), the Major State Basic Research Development Program (973 Program) (no. 2012CB722701), the Scientific Research Fund of Wuhan Textile University, the Natural Science Foundation for Young Scholar of Shanxi Province (no. 2013JQ6005), and the Scholarship Award for Excellent Doctoral Student granted by Ministry of Education of China (no. 1343-71134001002). Professor Xiaoqing Liu, Wuhan University of Technology, is acknowledged for help in the transmission electron microscopy JEOL 2100F testing.References
- M. Armand and J. M. Tarascon, Nature, 2008, 451, 652 CrossRef CAS PubMed.
- D. Deng, M. G. Kim, J. Y. Lee and J. Cho, Energy Environ. Sci., 2009, 2, 818 CAS.
- D. L. Ma, Z. Y. Cao, H. G. Wang, X. L. Huang, L. M. Wang and X. B. Zhang, Energy Environ. Sci., 2012, 5, 8538 CAS.
- H. G. Wang, D. L. Ma, X. L. Huang, Y. Huang and X. B. Zhang, Sci. Rep., 2012, 2, 701 Search PubMed.
- X. L. Huang, R. Z. Wang, D. Xu, Z. L. Wang, H. G. Wang, J. J. Xu, Z. Wu, Q. C. Liu, Y. Zhang and X. B. Zhang, Adv. Funct. Mater., 2013, 23(35), 4274 CrossRef CAS.
- P. G. Bruce, B. Scrosati and J. M. Tarascon, Angew. Chem., Int. Ed., 2008, 47, 2930 CrossRef CAS PubMed.
- C. F. Zhang, Z. X. Chen, Z. P. Guo and X. W. (David) Lou, Energy Environ. Sci., 2013, 6, 974 CAS.
- Y. S. Hu, X. Liu, J. O. Muller, R. Schlogl, J. Maier and D. S. Su, Angew. Chem., Int. Ed., 2009, 48, 210 CrossRef CAS PubMed.
- C. K. Chan, H. Peng, R. D. Twesten, K. Jarausch, X. F. Zhang and Y. Cui, Nano Lett., 2007, 7, 490 CrossRef CAS PubMed.
- A. M. Glushenkov, V. I. Stukachev, M. F. Hassan, G. G. Kuvshinov, H. K. Liu and Y. Chen, Cryst. Growth Des., 2008, 8, 3661 CAS.
- H. B. Wu, A. Q. Pan, H. H. Hng and X. W. (David) Lou, Adv. Funct. Mater., 2013, 23, 5669 CrossRef CAS.
- Y. Wang, K. Takahashi, K. Lee and G. Z. Cao, Adv. Funct. Mater., 2006, 16, 1133 CrossRef CAS.
- Z. L. Wang, D. Xu, L. M. Wang and X. B. Zhang, ChemPlusChem, 2012, 77, 124 CrossRef CAS.
- M. Gotić, S. Popović, M. Ivanda and S. Musić, Mater. Lett., 2003, 57, 3186 CrossRef.
- S. Q. Wang, S. R. Li, Y. Sun, X. Y. Feng and C. H. Chen, Energy Environ. Sci., 2011, 4, 2854 CAS.
- Y. Yang, S. P. Albu, D. Kim and P. Schmuki, Angew. Chem., Int. Ed., 2011, 50, 9071 CrossRef CAS PubMed.
- H. G. Wang, D. L. Ma, Y. Huang and X. B. Zhang, Chem.–Eur. J., 2012, 18, 8987 CrossRef CAS PubMed.
- X. Y. Chen, E. Pomerantseva, P. Banerjee, K. Gregorczyk, R. Ghodssi and G. Rubloff, Chem. Mater., 2012, 24, 1255 CrossRef CAS.
- K. Takahashi, S. J. Limmer, Y. Wang and G. Z. Cao, J. Phys. Chem. B, 2004, 108, 9795 CrossRef CAS.
- K. Takahashi, Y. Wang and G. Z. Cao, Appl. Phys. Lett., 2005, 86, 053102 CrossRef PubMed.
- J. Liu, X. Wang, Q. Peng and Y. Li, Adv. Mater., 2005, 17, 764 CrossRef CAS.
- G. Gu, M. Schmid, P. Chiu, A. Minett, J. Fraysse, G. Kim, S. Roth, M. Kozlov, E. Munoz and R. H. Bauhgman, Nat. Mater., 2003, 2, 316 CrossRef CAS PubMed.
- H. M. Liu and W. S. Yang, Energy Environ. Sci., 2011, 4, 4000 CAS.
- S. Lu, L. Hou and F. Gan, Thin Solid Films, 1999, 353, 40 CrossRef CAS.
- T. D. Nguyen and T. O. Do, Langmuir, 2009, 25, 5322 CrossRef CAS PubMed.
- X. F. Zhang, K. X. Wang, X. Wei and J. S. Chen, Chem. Mater., 2011, 23, 5290 CrossRef CAS.
- A. Pan, H. B. Wu, L. Yu and X. W. Lou, Angew. Chem., Int. Ed., 2013, 52, 2226 CrossRef CAS PubMed.
- J. Geder, H. E. Hoster, A. Jossen, J. Garche and D. Y. W. Yu, J. Power Sources, 2014, 257, 286 CrossRef CAS PubMed.
- A. Q. Pan, H. B. Wu, L. Zhang and X. W. (David) Lou, Energy Environ. Sci., 2013, 6, 1476 CAS.
- S. Q. Wang, Z. D. Lu, D. Wang, C. G. Li, C. H. Chen and Y. D. Yin, J. Mater. Chem., 2011, 21, 6365 RSC.
- J. Liu, Y. C. Zhou, C. P. Liu, J. B. Wang, Y. Pan and D. F. Xue, CrystEngComm, 2012, 14, 2669 RSC.
- S. Bai and X. P. Shen, RSC Adv., 2012, 2, 64 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, XRD pattern, XPS survey spectrum and high-resolution V2p3/2 XPS spectra of the as-prepared sample. TG-DSC curves of the NH4V4O10 powders in air atmosphere with a heating rate of 10 °C min−1; SEM images of four hydrothermally prepared samples: 30 min, 1 h, 3 h and 5 h. Schematic illustration of the formation process of NH4V4O10 microspheres from the side view. XRD pattern of hairy ball-like V2O5 spheres after annealing at 360, 400 and 500 °C. Low-magnification SEM images of hairy ball-like V2O5 spheres after annealing at different temperatures: 360; 400; and 500 °C. During the cycles, the coulombic efficiency of V2O5 spheres after annealing at different temperatures. See DOI: 10.1039/c4ra04259j |
| This journal is © The Royal Society of Chemistry 2014 |