Synthesis of copper nanoparticles by a T-shaped microfluidic device
Abstract
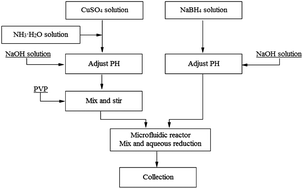
The copper nanoparticles were prepared by reduction of metal salt solutions with sodium borohydride in a T-shaped microfluidic device at room temperature. The influence of flow rates on copper particle diameter, morphology, size distribution, and elemental compositions has been investigated. Experimental results demonstrated that copper nanoparticles were uniform in size distribution, without being oxidized. With the increase in fluid flow rate the copper nanoparticles mean diameter increased, and the surface plasmon resonance absorptions of copper nanoparticles exhibited slight blue-shifting.

 Please wait while we load your content...
Please wait while we load your content...