Hydrogen bond donor–acceptor–donor organocatalysis for conjugate addition of benzylidene barbiturates via complementary DAD–ADA hydrogen bonding†
Abstract
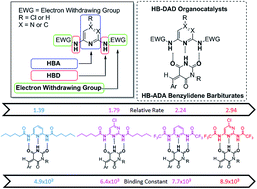
A new class of hydrogen bond donor–acceptor–donor (HB-DAD) organocatalysts has been developed for conjugate addition of benzylidene barbiturates. HB-DAD organocatalyst 1a (featuring para-chloro-pyrimidine as the hydrogen bond acceptor (HBA), N–H as the hydrogen bond donor (HBD) and a trifluoroacetyl group as the electron withdrawing group (EWG)) is able to activate benzylidene barbiturates through complementary DAD–ADA hydrogen bonding. Using 1a in benzylidene barbiturate conjugate addition, good yields were achieved. The relative rate constant (krel = 2.9) of 1a in catalyzing the conjugate addition of benzylidene barbiturates and the binding constant (KA = 8936 (±723) M−1) of 1a with benzylidene barbiturates were determined by NMR and UV/Vis. spectroscopy studies. The excellent correlation (R2 = 0.97) between the relative rate constant and binding affinity of 1a with benzylidene barbiturates provides support for the importance of DAD–ADA hydrogen bonding in organocatalysis.

 Please wait while we load your content...
Please wait while we load your content...