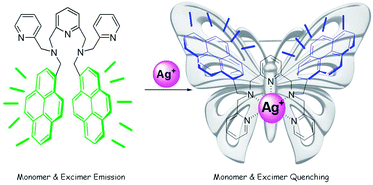
Pyrene pyridine-conjugate as Ag selective fluorescent chemosensor†
K. Velmurugana,
A. Ramanb,
S. Easwaramoorthi*b and
R. Nandhakumar*a
aDepartment of Chemistry, Karunya University, Coimbatore-641 114, India. E-mail: nandhakumar@karunya.edu
bChemical Laboratory, CSIR-Central Leather Research Institute, Adyar, India. E-mail: moorthi@clri.res.in
First published on 23rd July 2014
Abstract
A new pyrene pyridine conjugate (PPC) has been developed as a selective fluorescent sensor for Ag+ ion. Probe PPC exhibits high selectivity and sensitivity toward Ag+ as fluorescence ‘on–off’ behaviour in HEPES-buffered DMSO–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, (v/v) HEPES = 50 mM, pH = 7.4) solution with a detection limit of 0.29 × 10−8 M−1. The turn-off fluorescence sensing of the Ag+ ion occurs through dual pathways, i.e. changes and alternations in the photophysical properties. The binding of Ag+ ion prevents excimer formation and also induces intramolecular photoinduced electron transfer (PET) from the pyridine–Ag+ ion bound receptor to pyrene because of structural rigidification, which quenches excimer and monomer emissions with a butterfly-like skeleton.
1, (v/v) HEPES = 50 mM, pH = 7.4) solution with a detection limit of 0.29 × 10−8 M−1. The turn-off fluorescence sensing of the Ag+ ion occurs through dual pathways, i.e. changes and alternations in the photophysical properties. The binding of Ag+ ion prevents excimer formation and also induces intramolecular photoinduced electron transfer (PET) from the pyridine–Ag+ ion bound receptor to pyrene because of structural rigidification, which quenches excimer and monomer emissions with a butterfly-like skeleton.
1. Introduction
The development of metal-selective fluorescent chemosensors for sensing heavy and transition metal ions (HTMs) are continually receiving increasing attention by researchers because these metal ions show severe toxicity to living organisms, including humans.1,2 Silver ions have been utilized in killing harmful bacteria,3 and its complexes find extensive applications in electric, photographic, imaging, and pharmaceutical industries. Although, the overuse of medication containing silver salts results in the accumulation of silver in liver tissues; its adverse effects on patients has caused potential concerns.4,5 Therefore, detecting trace levels of silver is of significant importance. Over the years, several developmental studies focused on chemical sensors for Ag+.6 However, not many reports are available for the detection of Ag+ when compared to that of other heavy metal ions such as mercury and cadmium. Indeed, almost all the reported sensors for Ag+ still suffer from serious drawbacks such as poor water solubility, poor sensitivity, and low selectivity.7 As a consequence, the design and synthesis of a selective and sensitive fluorescent chemosensor for Ag+ is still highly desirable.Traditional analytical methods used for the trace determination of Ag+ ion are generally based on different instrumental techniques.8 Fluorescent probes are the prevailing tools for monitoring biologically relevant species in vitro/in vivo because of their simplicity and high sensitivity.9 So far, Ag+-ion reported probes have been classified according to their molecular structures as cyclic N, O, S-donor crown ethers,10 acyclic N, O, S-donor crown ethers,11 excimer,12 Ag+–π interaction,13 porphyrin or phthalocyanine14 and polymers, quantum dots, nanoparticles, and DNAs or oligonucleotides.15 As a continuation of our previous work,16 we herein report a new pyrene pyridine conjugate (PPC) bearing two pyrene and three pyridine groups as a selective fluorescent sensor for Ag+ ion. Probe PPC shows fluorescence quenching selectively for Ag+ ions by the intramolecular photoinduced electron transfer mechanism assisted by the excimer-monomer emissions of the pyrenes at a physiological pH of 7.4. A possible mechanism is proposed for the interaction of PPC with Ag+ through fluorescence changes, NMR experiments, mass spectrum and theoretical calculations.
2. Results and discussion
The receptor 1,1′-(pyridine-2,6-diyl)bis(N-(pyren-1-ylmethyl)-N-(pyridin-2-ylmethyl)methanamine), PPC, was conveniently synthesized from pyrene carboxaldehyde through a two-step protocol, as shown in Scheme 1. The reaction between pyrene carboxaldehyde (3) and pyridine-2-methylamine in a co-solvent of ethanol and chloroform followed by sodium borohydride reduction yielded compound 2. Subsequently, compound 2 and 2,6-bis(bromomethyl)pyridine were reacted in the presence of potassium carbonate in acetonitrile. Silica gel column chromatography afforded the designed receptor PPC in moderate yields. The structure of all the compounds was confirmed by NMR and mass spectral analysis (Fig. S1–S6†). PPC is designed in such a manner that the reporter unit pyrene is covalently attached to the receptor unit, the pyridyl moiety, through saturated, flexible, sp3 hybridized carbon. This feature enables controlling the distance or intramolecular interaction between the pyrene units by geometrical changes accompanied upon the binding of metal ions with the pyridine scaffold.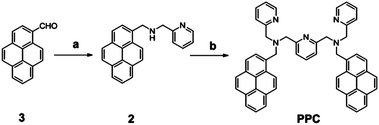 | ||
| Scheme 1 Synthesis of compound PPC (a) (i) pyridine-2-methylamine, EtOH/CHCl3, reflux, 3 h, (ii) EtOH, NaBH4, 3 h, rt. (b) 2,6-bis(bromomethyl)pyridine, K2CO3, CH3CN, reflux, 12 h. | ||
The UV-visible absorption spectrum of PPC measured in DMSO–H2O, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
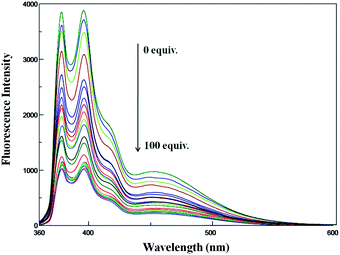
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, HEPES = 50 mM, pH = 7.4 (ESI, Fig. S7†) shows features similar to that of pyrene, and the spectral features are found to be independent of the concentration used in these studies. The fluorescence spectrum of PPC (Fig. 1) displayed both a typical intramolecular excimer emission around 453 nm and monomer emissions around 378 and 396 nm in DMSO–H2O (1
1 v/v, HEPES = 50 mM, pH = 7.4 (ESI, Fig. S7†) shows features similar to that of pyrene, and the spectral features are found to be independent of the concentration used in these studies. The fluorescence spectrum of PPC (Fig. 1) displayed both a typical intramolecular excimer emission around 453 nm and monomer emissions around 378 and 396 nm in DMSO–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, (v/v) HEPES = 50 mM, pH = 7.4). The excimer emission band is attributed to the interaction of two intramolecular pyrene units through a π–π interaction, which is fixed by the pyridine scaffold.17 Then, the cation-binding property of PPC was investigated using fluorescence spectroscopy by the addition of metal ions, such as Na+, K+, Al3+, Cu2+, Cd2+, Hg2+, Zr2+, Pb2+, Zn2+, Co2+, Ni2+, Ca2+, Mn2+, Cr3+, Ba2+, Ce2+, Mg2+, Fe2+, Fe3+ and Ag+, in aqueous solution. The fluorescence spectra of PPC (4 × 10−6 M) in the presence of different metal ions, given in Fig. 1, reveal that the fluorescence intensity becomes suppressed to a smaller but significant extent, characteristic to that of the metal ions (100 equiv.) added to the receptor solution. Indeed, significant amount of PPC fluorescence quenching due to the addition of Ag+ ions (100 equiv.) suggests that PPC can exclusively recognize Ag+ ion over the other cations, including the paramagnetic ones, via a fluorescence turn-off mechanism.
1, (v/v) HEPES = 50 mM, pH = 7.4). The excimer emission band is attributed to the interaction of two intramolecular pyrene units through a π–π interaction, which is fixed by the pyridine scaffold.17 Then, the cation-binding property of PPC was investigated using fluorescence spectroscopy by the addition of metal ions, such as Na+, K+, Al3+, Cu2+, Cd2+, Hg2+, Zr2+, Pb2+, Zn2+, Co2+, Ni2+, Ca2+, Mn2+, Cr3+, Ba2+, Ce2+, Mg2+, Fe2+, Fe3+ and Ag+, in aqueous solution. The fluorescence spectra of PPC (4 × 10−6 M) in the presence of different metal ions, given in Fig. 1, reveal that the fluorescence intensity becomes suppressed to a smaller but significant extent, characteristic to that of the metal ions (100 equiv.) added to the receptor solution. Indeed, significant amount of PPC fluorescence quenching due to the addition of Ag+ ions (100 equiv.) suggests that PPC can exclusively recognize Ag+ ion over the other cations, including the paramagnetic ones, via a fluorescence turn-off mechanism.
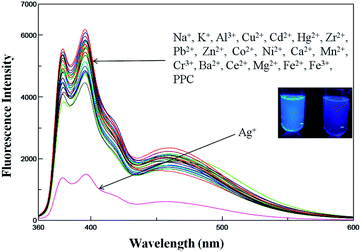 | ||
Fig. 1 Fluorescence changes of PPC (4 × 10−6 M) solution (DMSO–H2O, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, HEPES = 50 mM, pH = 7.4) in the presence of various metal ions (100 equiv. of each, excited at 348 nm). 1 v/v, HEPES = 50 mM, pH = 7.4) in the presence of various metal ions (100 equiv. of each, excited at 348 nm). | ||
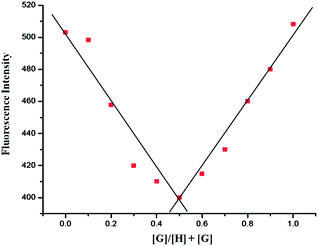
To understand the sensing behaviour of the Ag+ ion by PPC at the molecular level, fluorescence titration experiments were performed and the spectra is shown in Fig. 2. Upon incremental addition of Ag+ (0–100 equiv.) to the PPC solution, the fluorescence emission intensity was quenched gradually and reached the saturation level when 100 equiv. of Ag+ ions was employed. The stoichiometry for PPC and Ag+ was found to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 based on the electrospray ionization (ESI) mass spectrum (Fig. S6†) and the Job's method of continuous variation (Fig. 3).18 A significant peak at m/z 858.3, which pertains to [PPC + Ag]+ was clearly noticed in the mass spectrum. On the basis of the titration profile, the association constant (Ka)19 for PPC + Ag+ was determined to be 1.60 × 104 M−1 using the Benesi–Hildebrand equation (Fig. 4).20 The extrapolation of the plot suggests that the lowest possible detection limit of Ag+ by PPC was determined to be 0.29 × 10−8 M−1, (Fig. S8†), which is significantly higher than the allowed Ag+ concentration in drinking water of 0.05 mg L.
1 based on the electrospray ionization (ESI) mass spectrum (Fig. S6†) and the Job's method of continuous variation (Fig. 3).18 A significant peak at m/z 858.3, which pertains to [PPC + Ag]+ was clearly noticed in the mass spectrum. On the basis of the titration profile, the association constant (Ka)19 for PPC + Ag+ was determined to be 1.60 × 104 M−1 using the Benesi–Hildebrand equation (Fig. 4).20 The extrapolation of the plot suggests that the lowest possible detection limit of Ag+ by PPC was determined to be 0.29 × 10−8 M−1, (Fig. S8†), which is significantly higher than the allowed Ag+ concentration in drinking water of 0.05 mg L.
Most of the sensors based on two pyrene units covalently linked through a common binding unit, generally show ratiometric fluorescence response because of the changes in the ratio of excimer-monomer emissions caused by the difference in the conformational changes before and after binding. To our surprise, binding of Ag+ ion through the coordination of three ‘N’ atoms of the pyridine rings in PPC occurred through the quenching of both the excimer and monomer emission. The Ag+ coordination with the pyridine scaffold resulted in the rigidification of PPC and inhibits the π–π stacking by forcing the pyrene groups to move far away from each other and preventing the formation of an excimer.21 To confirm this hypothesis we optimized the geometries of PPC and PPC–Ag+ at B3LYP/6-31G* level of theory using Gaussian 03 software,22 and the figures are given in the ESI.† The energy minimized geometry of PPC reveals that both the pyrene moieties are oriented diagonal to each other, and are in fact held covalently by the flexible pyridine scaffold. The molecular flexibility enables the PPC to form an intramolecular excimer upon photoexcitation. However, after Ag+ ion binding with the pyridine scaffold, the PPC shows a butterfly-like skeleton and both the pyrene units are maintained far away from each other by the rigid pyridine–Ag+ moiety (Fig. S9–S12†). Thus, the binding of Ag+ ions prevents the formation of the excimer and thereby causes the quenching of excimer fluorescence when compared to the free PPC. On the other hand, monomer emission quenching can be explained by the heavy atom effect of Ag+, and preferably by the intramolecular photoinduced electron transfer mechanism (PET)23 from the nitrogen atoms of the pyridine ring to the pyrene unit.24 PET was possible because of the rigidification of the pyridine scaffold, which aids in the electron transfer process. The hypothesis that the electron density is redistributed from the pyridine–Ag+ scaffold to pyrene during transition from the highest occupied molecular orbitals (HOMO) to the lowest unoccupied molecular orbital (LUMO) (Fig. S12†) is also confirmed by theoretical calculations.
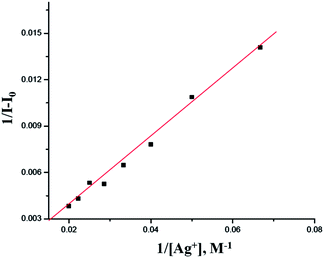 | ||
Fig. 4 Benesi–Hildebrand plot of PPC, assuming 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry for the association between PPC and Ag+. 1 stoichiometry for the association between PPC and Ag+. | ||
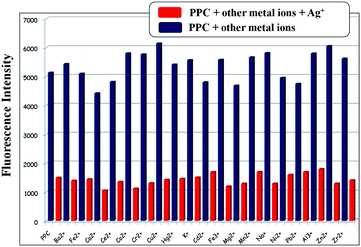
To better investigate the practical applicability of PPC as an Ag+ ion selective fluorescent sensor, competitive experiments were carried out in the presence of Ag+ ion (100 equiv.) mixed with Na+, K+, Al3+, Cu2+, Cd2+, Hg2+, Zr2+, Pb2+, Zn2+, Co2+, Ni2+, Ca2+, Mn2+, Cr3+, Ba2+, Ce2+, Mg2+, Fe2+ and Fe3+ at 100 equiv., as shown in Fig. 5. No significant interference in the detection of Ag+ with the sensor PPC was observed in the presence of most competitive metal ions, for which interference with the detection signal abolished the quenching effect on the binding of Ag+ ion. Accordingly, these observations suggested that the sensor PPC can be used as selective fluorescent sensors for Ag+ ion in the presence of most competitive metal ions.
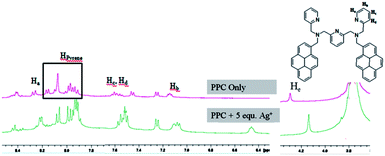
Furthermore, 1H NMR titration experiment was performed to understand the nature of interactions between the PPC and the Ag+ ion, and the selected spectra are given in Fig. 6. The comparison of the 1H NMR spectra of PPC and PPC mixed with 10 equiv. of Ag+ ion suggest that the addition of Ag+ to the PPC solution led to an upfield shift of the aromatic protons on the pyridine rings. In these NMR-titration spectra, pyridine Ha, Hb, Hc, Hd-protons shifted from δ 8.25–8.27 to 8.20–8.22 ppm, 7.12–7.15 to 7.06–7.10 ppm, 7.58–7.62 to 6.44–6.47 ppm, 7.53–7.55 to 7.49–7.51 ppm, respectively, and pyridine methylene protons shifted from δ 4.29 to 4.12 ppm. On the addition of Ag+ to PPC, the pyridine groups come closer together during coordination, and hence there are notable wide ranges of upfield chemical shifts, which was also confirmed by theoretical calculations.16
In addition, the SEM images of PPC and PPC + Ag+ are displayed in Fig. 7. The morphological change after silver insertion was confirmed by SEM analysis. The receptor PPC shows a large molecular size in the free state. However, upon the addition of Ag+, the size of the complex molecule compared with PPC becomes smaller and agglomerated because of the formation of the PPC + Ag+ coordination complex, which further confirms the selectivity of the probe PPC toward Ag+ ions.
On the basis of NMR and theoretical studies, the plausible binding mechanism of PPC in the present system is schematically depicted in Scheme 2. The ‘N’ of the three pyridine rings, and pyridine methylamine of the two ‘N’ are engaged in complexation with Ag+-ion.
A sensing system with real applications is important to monitor the Ag+ concentration in real samples. In addition to prove the viability of the Ag+ sensing system using PPC, we tested Ag+ in Karunya Nagar tap and waste water samples with a variety of interferences. Insoluble substances were removed from the tap and waste water samples by membrane filtration. The tap water was spiked with target Ag+ at two different concentration levels of 4 and 8 ppm and without spiking of Ag+ in waste water samples, the concentrations were 9 and 13 ppm. The sensing and recoveries of Ag+ from four water samples were from 97% to 100%, as shown in Table 1. The above mentioned results show that the PPC can be used for the detection of Ag+ in real samples.
| Sample | Amount of Ag+ present in blank ppm (AAS) | Ag+-ion spiked (ppm) | Ag+-ion found (ppm) (fluorescence) (mean ± S.D.) | Recovery (%) |
|---|---|---|---|---|
| Tap water 1 | 0.12 | 4 | 4.03 ± 0.03 | 97 |
| Tap water 2 | 0.28 | 8 | 8.20 ± 0.08 | 99 |
| Waste water 1 | 9.17 | 0 | 9.22 ± 0.15 | 100 |
| Waste water 2 | 13.84 | 0 | 13.87 ± 0.02 | 100 |
3. Experimental methods
Synthesis of compound 2
Pyrene carboxaldehyde (1 g, 4.4 mmol) and pyridine-2-methylamine (0.52 g, 4.8 mmol) were dissolved in a mixture of solvents: absolute ethanol (20 mL) and chloroform (10 mL). The mixture was heated under reflux for 3 h and after cooling to room temperature, the precipitate was separated and washed with ethanol to afford the crude Schiff base. The Schiff base was combined with NaBH4 (0.17 g, 4.4 mmol) and ethanol (20 mL). The resulting reaction mixture was stirred at room temperature for 3 h. The solvent was then removed and methylene chloride (30 mL) and HCl (0.2 N, 30 mL) were added. After stirring for 1 h, the organic layer was separated and washed with brine and then dried over Na2SO4. The crude product was purified by a short silica gel column, and eluted with ethylacetate–methanol (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford the desired oily product 2.16 Yield: 1.15 g (82%). 1H NMR (CDCl3, 250 MHz): δ (ppm): 8.61 (d, 1H), 8.35 (d, 1H), 8.18–7.98 (m, 8H), 7.59 (t, 1H), 7.31 (d, 1H), 7.15 (t, 1H), 4.52 (s, 2H), 4.09 (s, 2H), 2.46 (s, 1H). 13C NMR (CDCl3, 62.9 MHz): δ (ppm): 51.33, 54.62, 55.05, 55.45, 76.68, 77.19, 77.39, 77.69, 121.98, 122.42, 123.32, 124.69, 124.98, 125.01, 125.04, 125.84, 127.05, 127.09, 127.47, 127.55, 129.17, 130.68, 130.86, 131.32, 133.68, 136.43, 148.86, 149.30, 149.73, 159.86.
1) to afford the desired oily product 2.16 Yield: 1.15 g (82%). 1H NMR (CDCl3, 250 MHz): δ (ppm): 8.61 (d, 1H), 8.35 (d, 1H), 8.18–7.98 (m, 8H), 7.59 (t, 1H), 7.31 (d, 1H), 7.15 (t, 1H), 4.52 (s, 2H), 4.09 (s, 2H), 2.46 (s, 1H). 13C NMR (CDCl3, 62.9 MHz): δ (ppm): 51.33, 54.62, 55.05, 55.45, 76.68, 77.19, 77.39, 77.69, 121.98, 122.42, 123.32, 124.69, 124.98, 125.01, 125.04, 125.84, 127.05, 127.09, 127.47, 127.55, 129.17, 130.68, 130.86, 131.32, 133.68, 136.43, 148.86, 149.30, 149.73, 159.86.
Synthesis of PPC
Compound 2 (0.5 g, 1.66 mmol), K2CO3 (0.3 g, 2.22 mmol), and dry CH3CN (10 mL) were placed in a round-bottom flask at room temperature. To this mixture, a CH3CN solution (40 mL) of 2,6-bis(bromomethyl)pyridine (0.2 g, 0.75 mmol) was added dropwise over 30 min. The reaction mixture was then heated to reflux for 12 h. After cooling to room temperature, the insoluble materials were removed by filtration and washed with chloroform several times until the washing solvent became colorless. The combined filtrate was concentrated and the resulting residue was purified by silica gel column chromatography using ethylacetate–hexanes (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the eluent to give the final sensor PPC. Yield: 60%, Mp: 152 °C. 1H NMR (CDCl3, 250 MHz): δ (ppm): 8.52–8.54 (d, 2H), 8.34–8.38 (d, 2H), 7.91–8.13 (m, 18H), 7.53 (s, 4H), 7.06–7.35 (m, 3H), 6.75–6.77 (s, 2H), 4.42 (s, 4H). 13C NMR (DMSO, 125 MHz): δ (ppm): 57.22, 60.47, 60.64, 121.51, 121.96, 123.32, 124.07, 124.42, 124.67, 124.88, 124.90, 125.75, 126.96, 127.01, 127.21, 127.35, 128.24, 129.82, 130.69, 130.77, 131.22, 132.50, 136.58, 148.77, 158.62, 159.60. LC-MS m/z: calcd for [M+] 751; found [M + H]+ 752.
1) as the eluent to give the final sensor PPC. Yield: 60%, Mp: 152 °C. 1H NMR (CDCl3, 250 MHz): δ (ppm): 8.52–8.54 (d, 2H), 8.34–8.38 (d, 2H), 7.91–8.13 (m, 18H), 7.53 (s, 4H), 7.06–7.35 (m, 3H), 6.75–6.77 (s, 2H), 4.42 (s, 4H). 13C NMR (DMSO, 125 MHz): δ (ppm): 57.22, 60.47, 60.64, 121.51, 121.96, 123.32, 124.07, 124.42, 124.67, 124.88, 124.90, 125.75, 126.96, 127.01, 127.21, 127.35, 128.24, 129.82, 130.69, 130.77, 131.22, 132.50, 136.58, 148.77, 158.62, 159.60. LC-MS m/z: calcd for [M+] 751; found [M + H]+ 752.
4. Conclusions
In conclusion, we have developed a new fluorescent chemosensor, a bis-pyrene tethered pyridine scaffold for sensing Ag+ ion. The sensor exclusively senses Ag+ ion by quenching the fluorescence signals in two manners: the excimer emission is quenched by avoiding the interaction between the two intramolecular pyrene units and the monomer emission was quenched because of photoinduced electron transfer (PET) from the free tertiary amino nitrogen of pyridine–Ag+ ion bound receptor to pyrene. The fluorescent chemosensor was selective and sensitive for Ag+ and was capable of detecting the metal ion down to 0.29 × 10−8 M−1. The complex formation, stoichiometry, and binding modes have been established by fluorescence spectroscopy, 1H NMR studies, mass spectroscopy and theoretical calculation and were found to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The design strategy of the sensor and photophysical properties would be assisting in extending the development of several fluorescent chemosensors for heavy and transition metal ions.
1. The design strategy of the sensor and photophysical properties would be assisting in extending the development of several fluorescent chemosensors for heavy and transition metal ions.
Acknowledgements
This work is supported by the Fast Track Programme for the young Scientists of the DST (no. SR/FT/CS-95/2010). The work at CSIR-CLRI is supported by the XII-plan suprainstitutional project “STRAIT”.Notes and references
- (a) V. Amendola, L. Fabbrizzi, F. Forti, M. Licchelli, C. Mangano, P. Pallavicini, A. Poggi, D. Sacchi and A. Taglieti, Coord. Chem. Rev., 2006, 250, 273 CrossRef CAS PubMed; (b) B. Valeur and I. Leray, Coord. Chem. Rev., 2000, 205, 3 CrossRef CAS; (c) A. P. DeSilva, D. B. Fox, A. J. M. Huxley and T. S. Moody, Coord. Chem. Rev., 2000, 205, 41 CrossRef CAS.
- (a) T. C. Hutchinson and K. M. Meema, Lead, Mercury, Cadmium and Arsenic in the Environment, Wiley, New York, 1987, p. 6987 Search PubMed; (b) I. Onyido, A. R. Norris and E. Buncel, Chem. Rev., 2004, 104, 5911 CrossRef CAS PubMed.
- M. Yamanaka, K. Hara and J. Kudo, Appl. Environ. Microbiol., 2005, 71, 7589 CrossRef CAS PubMed.
- (a) M. Alvarez-Corral, M. Dorado and I. Rodriguez-Garcia, Chem. Rev., 2008, 108, 3174 CrossRef CAS PubMed; (b) L. Chopra, J. Antimicrob. Chemother., 2007, 59, 587 CrossRef PubMed; (c) M. Kazuyuki, H. Nobuo, K. Takatoshi, K. Yuriko, H. Osamu, I. Yashihisa and S. Kiyoko, Clin. Chem., 2001, 47, 763 Search PubMed.
- X.-B. Zhang, Z.-X. Han, Z.-H. Fang, G.-L. Shen and R.-Q. Yu, Anal. Chim. Acta, 2006, 562, 210 CrossRef CAS PubMed.
- (a) I. T. Ho, K. C. Haung and W. S. Chung, Chem.–Asian J., 2011, 6, 2738 CrossRef CAS PubMed; (b) Z. Xu, S. Zheng, J. Yoon and D. R. Spring, Analyst, 2010, 135, 2554 RSC; (c) C. Zhao, K. Qu, Y. Song, C. Xu, J. Ren and X. Qu, Chem.–Asian J., 2010, 16, 8147 CAS; (d) H. H. Wang, L. Xue, Y. Y. Qian and H. Jiang, Org. Lett., 2010, 12, 292 CrossRef CAS PubMed; (e) K. M. K. Swamy, H. N. Kim, J. H. Soh, Y. Kim, S.-J. Kim and J. Yoon, Chem. Commun., 2009, 1234 RSC; (f) A. Chatterjee, M. Santra, N. Won, S. Kim, J. K. Kim, S. B. Kim and K. H. Ahn, J. Am. Chem. Soc., 2009, 131, 2040 CrossRef CAS PubMed; (g) R. Joseph, B. Ramanujam, A. Acharya and C. P. J. Rao, Org. Chem., 2009, 74, 8181 Search PubMed; (h) L. Liu, D. Zhang, G. Zhang, J. Xiang and D. Zhu, Org. Lett., 2008, 10, 2271 CrossRef CAS PubMed; (i) L. Liu, D. Zhang, G. Zhang, J. Xiang and D. Zhu, Org. Lett., 2008, 10, 2271 CrossRef CAS PubMed; (j) L. Liu, G. Zhang, J. Xiang, D. Zhang and D. Zhu, Org. Lett., 2008, 10, 4581 CrossRef CAS PubMed; (k) S. Iyoshi, M. Taki and Y. Yamamoto, Inorg. Chem., 2008, 47, 3946 CrossRef CAS PubMed; (l) X. Zhu, S. Fu, W. K. Wong and W. Y. Wong, Tetrahedron Lett., 2008, 49, 1843 CrossRef CAS PubMed; (m) J. Y. Lee, J. Kwon, C. S. Park, J. E. Lee, W. Sim, J. S. Kim, J. Seo, I. Yoon, J. H. Jung and S. S. Lee, Org. Lett., 2007, 9, 493 CrossRef CAS PubMed; (n) A. Coskun and E. U. Akkaya, J. Am. Chem. Soc., 2005, 127, 10464 CrossRef CAS PubMed; (o) M. Hu, J. Fan, J. Cao, K. Song, H. Zhang, S. Sun and X. Peng, Analyst, 2012, 137, 2107 RSC.
- J. F. Zhang, Y. Zhou, J. Yoon and J. S. Kim, Chem. Soc. Rev., 2011, 40, 3416 RSC.
- (a) A. Ceresa, A. Radu, S. Peper, E. Bakker and E. Pretsch, Anal. Chem., 2002, 74, 4027 CrossRef CAS; (b) K. Kimura, S. Yajima, K. Tatsumi, M. Yokoyama and M. Oue, Anal. Chem., 2000, 72, 5290 CrossRef CAS; (c) M. Mazloum, M. S. Niassary, S. H. M. Chahooki and M. K. Amini, Electroanalysis, 2002, 14, 376 CrossRef CAS; (d) R. K. Shervedani and M. K. Babadi, Talanta, 2006, 69, 741 CrossRef CAS PubMed; (e) M. Javanbakht, M. R. Ganjali, P. Norouzi, A. Badiei and A. Hasheminasab, Electroanalysis, 2007, 19, 1307 CrossRef CAS PubMed; (f) S. Chung, W. Kim, S. B. Park, I. Yoon, S. S. Lee and D. D. Sung, Chem. Commun., 1997, 965 RSC; (g) F. Teixidor, M. A. Flores, L. Escriche, C. Vinas and J. Casabo, Chem. Commun., 1994, 963 RSC; (h) X. Zeng, L. Weng, L. Chen, X. Leng, H. Ju, X. He and Z. Z. Zhang, J. Chem. Soc., Perkin Trans. 2, 2001, 545 RSC; (i) A. R. Zanganeh and M. K. Amini, Electrochim. Acta, 2007, 52, 3822 CrossRef CAS PubMed; (j) M. R. Bryce, B. Johnston, R. Kataky and K. Toth, Analyst, 2000, 125, 861 RSC.
- (a) C. W. Yu, J. Zhang, R. Wang and L. X. Chen, Org. Biomol. Chem., 2010, 8, 5277 RSC; (b) M. Beija, C. A. M. Afonso and J. M. G. Martinho, Chem. Soc. Rev., 2009, 38, 2410 RSC; (c) R. Wang, C. W. Yu, F. B. Yu and L. X. Chen, TrAC, Trends Anal. Chem., 2010, 29, 1004 CrossRef CAS PubMed; (d) J. Zhang, C. W. Yu, S. Y. Qian, G. Lu and J. L. Chen, Dyes Pigm., 2012, 92, 1370 CrossRef CAS PubMed; (e) B. X. Gao, Y. L. Liu, H. J. Yin, Y. X. Li, Q. Q. Bai and L. C. Zhang, New J. Chem., 2012, 36, 28 RSC; (f) Z. X. Xie, K. Wang, C. L. Zhang, Z. H. Yang, Y. C. Chen, Z. J. Guo, G. Y. Lu and W. J. He, New J. Chem., 2011, 35, 607 RSC.
- (a) T. Chen, W. Zhu, Y. Xu, S. Zhang, X. Zhang and X. Qian, Dalton Trans., 2010, 39, 1316 RSC; (b) Z. Xu, S. Zheng, J. Yoon and D. R. Spring, Analyst, 2010, 135, 2554 RSC.
- (a) V. K. Bhardwaj, A. P. S. Pannu, N. Singh, M. S. Hundal and G. Hundal, Tetrahedron, 2008, 64, 5384 CrossRef CAS PubMed; (b) K. M. K. Swamy, H. N. Kim, J. H. Soh, Y. Kim, S. J. Kim and J. Yoon, Chem. Commun., 2009, 1234 RSC; (c) B. Zhang, J. Sun, C. Bi, G. Yin, L. Pu, Y. Shi and L. Sheng, New J. Chem., 2011, 35, 849 RSC.
- H. Zhang, L. Xie, W. Yan, C. He, X. Cao and C. Duan, New J. Chem., 2009, 33, 1478 RSC.
- (a) R. Rathore, V. J. Chebny and S. H. Abdelwahed, J. Am. Chem. Soc., 2005, 127, 8012 CrossRef CAS PubMed; (b) F. Wang, J. H. Moon, R. Nandhakumar, B. Kang, D. Kim, K. M. Kim, J. Y. Lee and J. Yoon, Chem. Commun., 2013, 49, 7228 RSC.
- M. Ikeda, T. Tanida, M. Takeuchi and S. Shinkai, Org. Lett., 2000, 2, 1803 CrossRef CAS PubMed.
- (a) A. Chatterjee, M. Santra, N. Won, S. Kim, J. K. Kim, S. B. Kim and K. H. Ahn, J. Am. Chem. Soc., 2009, 131, 2040 CrossRef CAS PubMed; (b) J. G. Liang, X. P. Ai, Z. K. He and D. W. Pang, Analyst, 2004, 129, 619 RSC; (c) F. Qu, J. Liu, H. Yan, L. Peng and H. Li, Tetrahedron Lett., 2008, 49, 7438 CrossRef CAS PubMed; (d) H. Tong, L. Wang, X. Jing and F. Wang, Macromolecules, 2002, 35, 7169 CrossRef CAS; (e) A. Alizadeh, M. M. Khodaei, Z. Hamidi and M. B. Shamsuddin, Sens. Actuators, B, 2014, 190, 782 CrossRef CAS PubMed; (f) C. Zhao, K. Qu, Y. Song, C. Xu, J. Ren and X. Qu, Chem.–Eur. J., 2010, 16, 8147 CrossRef CAS PubMed; (g) Y. Wei, B. Li, X. Wang and Y. Duan, Biosens. Bioelectron., 2014, 58, 276 CrossRef CAS PubMed.
- F. Wang, R. Nandhakumar, J. H. Moon, K. M. Kim, J. Y. Lee and J. Yoon, Inorg. Chem., 2011, 50, 2240 CrossRef CAS PubMed.
- (a) C. J. Broan, Chem. Commun., 1996, 699 RSC; (b) S.-Y. Park, J.-H. Yoon, C.-S. Hong, R. Souane, J.-S. Kim, S. E. Matthews and J. Vicens, J. Org. Chem., 2008, 73, 8212 CrossRef CAS PubMed; (c) S. Jang, P. Thirupathi, L. N. Neupane, J. Seong, H. Lee, W. I. Lee and K.-H. Lee, Org. Lett., 2012, 14, 4746 CrossRef CAS PubMed.
- L. Tang, N. Wang, Q. Zhang, J. Guo and R. Nandhakumar, Tetrahedron Lett., 2013, 54, 536 CrossRef CAS PubMed.
- (a) D. W. Cho, Y. H. Kim, S. G. Kang, M. Yoon and D. Kim, J. Chem. Soc., Faraday Trans. 2, 1996, 92, 29 RSC; (b) O. Stern, M. Volmer, P. Zeitschr, 1919, 20, 183.
- M. Shortreed, R. Kopelman, M. Kuhn and B. Hoyland, Anal. Chem., 1996, 68, 1414 CrossRef CAS.
- (a) X.-L. Ni, X. Zeng, C. Redshaw and T. Yamato, Tetrahedron, 2011, 67, 3248 CrossRef CAS PubMed; (b) K.-C. Chang, I.-H. Su, A. Senthilvelan and W.-S. Chung, Org. Lett., 2007, 9, 3363 CrossRef CAS PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery, T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, C. Gonzalez and J. A. Pople, Gaussian 03, Revision E.01, Gaussian, Inc., Wallingford CT, 2004 Search PubMed.
- (a) K.-C. Chang, I.-H. Su, A. Senthilvelan and W.-S. Chung, Org. Lett., 2007, 9, 3363 CrossRef CAS PubMed; (b) A. Ojida, Y. Mito-oka, M.-A. Inoue and I. Hamachi, J. Am. Chem. Soc., 2002, 124, 6256 CrossRef CAS PubMed; (c) M. Choi, M. Kim, K. D. Lee, K.-N. Han, I.-A. Yoon, H.-J. Chung and J. Yoon, Org. Lett., 2001, 3, 3455–3457 CrossRef CAS PubMed.
- M.-Y. Chae, X. M. Cherian and A. W. Czarnik, J. Org. Chem., 1993, 58, 5797 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: general experimental methods; copies of 1H NMR, 13C NMR, and other optical spectra. See DOI: 10.1039/c4ra04001e |
| This journal is © The Royal Society of Chemistry 2014 |