iTRAQ-based proteomics for studying the effects of dioscin against nonalcoholic fatty liver disease in rats†
Lina Xua,
Yongli Weia,
Deshi Dongab,
Lianhong Yina,
Yan Qia,
Xu Hana,
Youwei Xua,
Yanyan Zhaoa,
Kexin Liua and
Jinyong Peng*ac
aCollege of Pharmacy, Dalian Medical University, 9 Western Lvshun South Road, Dalian 116044, China
bDepartment of Pharmaceuticals, The First Affiliated Hospital, Dalian Medical University, Dalian Medical University, Dalian 116011, China
cResearch Institute of Integrated Traditional and Western Medicine of Dalian Medical University, Dalian 116011, China. E-mail: jinyongpeng2005@163.com; Fax: +86 411 8611 0411; Tel: +86 411 8611 0411
First published on 3rd July 2014
Abstract
Dioscin shows protective effects against liver injury in our previous works. However, its activity against nonalcoholic fatty liver disease (NAFLD) and the mechanisms are still unknown. In this study, the effects of dioscin on high fat diet (HFD)-induced rats were tested, and one proteomic method was employed to investigate the possible mechanisms and biomarkers. The present work showed that dioscin showed significant hepatoprotective effects against NAFLD. Using iTRAQ labeling coupled with nano-LC-TOF-MS/MS analysis, a total of 449 altered proteins were found which connected with each other and were involved in different KEGG pathways based on bioinformatics analysis. Furthermore, 22 proteins which were involved in some important signaling pathways and may be the important biomarkers were validated by western blotting and quantitative real-time PCR assays. In conclusion, dioscin shows a significantly protective effect on NAFLD, and should be developed as a potential new drug for NAFLD treatment. Furthermore, our results not only provide new insights for treatment of NAFLD through affecting multiple drug targets and the cross-communication of many signal pathways, but also afford novel markers that may help to reveal the mechanisms of NAFLD, and prognostic assays and therapeutics for NAFLD.
Introduction
NAFLD encompasses a spectrum of liver disorders and is characterized by hepatocellular injury and the presence of inflammation with or without fibrosis, to end-stage of irreversible cirrhosis and hepatocellular carcinoma.1 The specific mechanism of NAFLD has not been fully elucidated but the “two-hit” theory2 has been widely accepted. Current research on NAFLD mainly focuses on fatty acid metabolism, bile secretion, oxidative phosphorylation, inflammation, glycolysis/gluconeogenesis3–6 and some signaling pathways including peroxisome proliferator-activated receptor (PPAR), phosphatidylinositol-3 kinase (PI3K)-Akt, mitogen-activated protein kinase (MAPK) and insulin signaling pathways7–10 are also involved. Development of NAFLD is very complex, and thus, an individual signal pathway or single biological process cannot comprehensively represent the pathogenesis and system of the disease. Thus, it is necessary to clearly elucidate the overall processes and signaling pathways of the disease and the mechanisms of chemicals for treatment of NAFLD. In recent years, proteomics as a powerful technology to find differentially expressed proteins and novel targets and bioinformatics as an essential supporting discipline for proteomic research have been widely used in life sciences.11–14 Some previously studies have been performed using proteomics techniques.15–17 However, there are no papers reporting the use of iTRAQ-based proteomics to assay rat liver samples for NAFLD investigation.Dioscin (Fig. 1A), a natural product, has anti-hyperlipidemic, anti-fungal, anti-virus and anti-cancer activities.18–21 Our previous investigations have shown that dioscin has remarkable hepatopro-tective activities against CCl4- and acetaminophen-induced acute liver damage in mice.22–24 However, there have been no papers to report the actions of dioscin against NAFLD to our best knowledge.
Results and discussion
Effects of dioscin on high-fat diet (HFD)-induced NAFLD rats
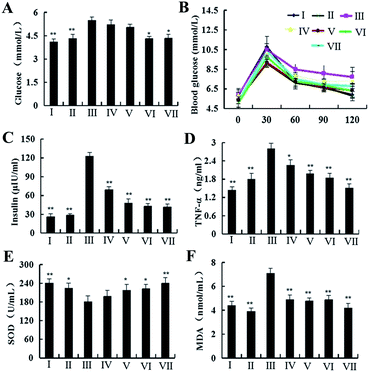
NAFLD, a complex disease, has been caused by the joint action of some genetic and environmental factors, and is associated with many clinical diseases. There are challenges and opportunities for the management and prevention of the disease. In our study, after feeding with HFD for 4 weeks, the body weights of the rats in model, positive control and dioscin-treated groups were increased significantly compared with normal and dioscin control groups (Fig. 1B). However, after treatment with dioscin (20, 40 and 60 mg kg−1) or silibinin for 12 weeks, the body weight gains of the rats in groups IV to VII were lower than that of in model group. Therefore, dioscin inhibited the obesity in HFD-treated rats.The effects of dioscin on the activities of serum AST and ALT in rats are shown in Fig. 1C. Compared with model group, the levels of AST and ALT were significantly decreased by dioscin. After treated by dioscin, the concentrations of leptin (Fig. 1D), the levels of TC, TG, LDL-Cand FFA (Fig. 1E–G) in blood were significantly decreased compared with model group, while the level of HDL-C (Fig. 1G) was markedly elevated. In addition, dioscin decreased the levels of glucose and insulin (Fig. 2A–C) compared with model group. All of these indicated that dioscin had obvious lipid- and glucose-lowering effects against NAFLD. In addition, the levels of SOD in livers (Fig. 2E) showed the significant decreases in HFD-treated rats compared with normal group. However, the rats administrated with dioscin at the dose of 60 mg kg−1 showed a markedly improvement compared with model group. As shown in Fig. 2D and F, significant increases of MDA and TNF-α levels were observed after the rats were exposed to HFD (p < 0.01), which were decreased by dioscin.
H&E and Sudan III stained sections are shown in Fig. 3. The histology of the liver was good in normal group, while the liver from model group showed widespread lipid vacuoles deposited inside the parenchyma cells. The rats treated with dioscin at the dose of 60 mg kg−1 showed less microvesicular fatty changes. Sudan III stained sections indicated that the red-stained regions were significantly decreased by dioscin (60 mg kg−1) compared with model group (Fig. 3).
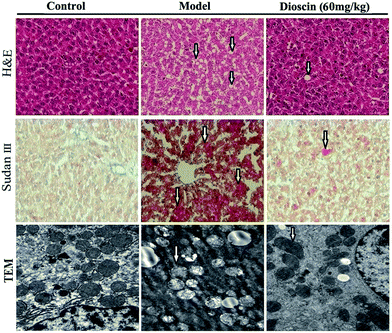 | ||
Fig. 3 Effects of dioscin on histopathological examination by H&E and Sudan III staining (100× magnification), and representative micrographs of TEM (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000× magnification) in rats. 000× magnification) in rats. | ||
TEM assay further revealed the ultrastructural alteration of mitochondria (shown in Fig. 3). Compared with normal rats, part of mitochondria valley disappearance was observed in model group, while dioscin (60 mg kg−1) markedly reversed mitochondrial injury caused by HFD challenge.
All of the results suggest that dioscin can improve insulin resistance, regulate oxidative stress, leptin and inflammatory cytokines to achieve the anti-NAFLD effect. For the excellent activities against the development of NAFLD, dioscin should be developed as a new drug for treatment of NAFLD.
Differentially expressed proteome in HFD-induced rats
The molecular mechanisms underlying NAFLD initiation and progression remain poorly understood due to the complexity of this biological process. Elucidation of the molecular events occurring is an essential step toward a more comprehensive understanding of NAFLD as well as the development of effective strategies for the diagnosis and treatment of this disease. Proteomics is an essential approach for elucidating the complex pathogenesis of NAFLD.In the present study, iTRAQ-based proteomic method was deployed to analyze the molecular mechanisms during NAFLD progress. This approach allowed us to select 449 differentially expressed proteins for further analysis. Of these proteins, 231 proteins were found to be up-regulated and 218 down-regulated in NAFLD rat model, respectively, suggesting a drastic phenotypic alteration during NAFLD model. The abbreviated lists of up- and down-regulated proteins are provided in ESI Table S1.†
Bioinformatics analysis of differentially expressed proteins
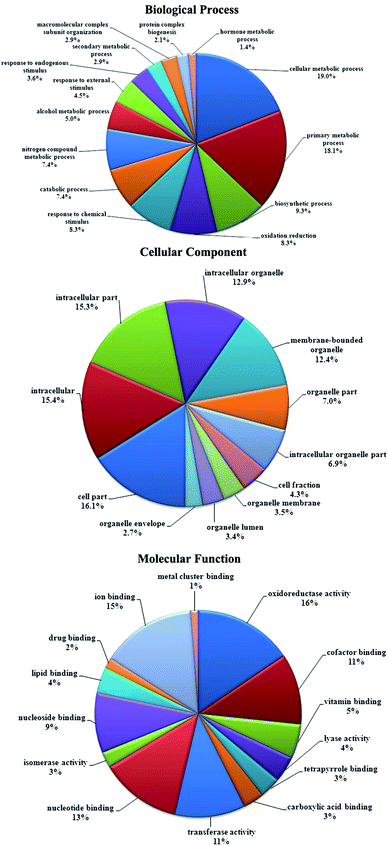
Functional classification of differentially expressed proteins from this study was performed according to gene ontology categories biological process (BP), cellular component (CC) and molecular function (MF). The results are shown in Fig. 4. A classification based on BP revealed that these proteins are mainly involved in cellular metabolic process (48.5%), primary metabolic process (46.1%), biosynthetic process (23.6%), oxidation reduction (21.2%), response to chemical stimulus (21.2%), catabolic process (18.8%), nitrogen compound metabolic process (18.8%) and alcohol metabolic process (12.7%). For CC, the top five subcategories are cell part (72.1%), intracellular (69.1%), intracellular part (68.5%), intracellular organelle (57.6%) and membrane-bounded organelle (55.8%). For MF, several major subcategories are oxidoreductase activity (23.0%), ion binding, nucleotide binding (21.8%), cofactor binding (18.8%), transferase activity (16.4%), nucleoside binding (16.4%), vitamin binding (13.9%), lyase activity (7.9%) and lipid binding (6.1%).Among these identified proteins, most of them were involved in biological pathways in KEGG, and 56 biological pathways contained more than 5 differentially expressed proteins. Some of the proteins belonged to multiple pathways. Among the pathways, fatty acid metabolism (10 proteins), bile secretion (8 proteins), primary bile acid biosynthesis (5 proteins), peroxisome (12 proteins), PPAR signaling pathway (9 proteins), PI3K-Akt signaling pathway (9 proteins), oxidative phosphorylation (8 proteins), MAPK signaling pathway (7 proteins), glycolysis/gluconeogenesis (7 proteins) and insulin signaling (6 proteins) pathway were the most remarkable and were differentially represented between NAFLD and normal rats. All the proteins involved in these pathways are provided in ESI Table S2,† and the representative pathway maps are given in ESI Fig. S1.†
To obtain a comprehensive view of the biological significance of differentially expressed proteins, these proteins were also categorized according to their main biological function by IPA. IPA calculated a significance score of each network, and the network with scores of 2 or higher has at least a 99% confidence of not being generated by random chance alone. Accordingly, 23 networks (score higher than 10) were found to be significant in the database (ESI Table S3 and Fig. S2†). Moreover, the proteins involved in the pathways and biological functions which we were interested in were put together and categorized again by IPA.
Effects of dioscin on the expressions of some proteins and genes
NAFLD is characterized by the increased intrahepatic triglyceride (hepatic steatosis). Steatosis is developed when the rate of fatty acids input is greater than output. Fatty acids in liver are derived from de novo synthesis and plasma-free fatty acids. Up-regulation of synthesis and/or uptake can result in fatty acid accumulation. Fatty acids in hepatocytes are metabolized by fatty acid oxidation and esterification to produce TG, which is either incorporated into lipoproteins or stored as lipid droplets within the hepatocytes. Defects in one or both of these pathways can lead to hepatic steatosis.25 Therefore, proteins involved in fatty acid metabolism were investigated. The expressions of Eci1, ACADS, ACLS5, ALDH7A1, ALDH2 and CYP4A2 were down-regulated, and Acadvl and Adh1 were up-regulated in model group compared with normal rats, which were all improved by dioscin except Eci1 (ESI Fig. S3 and S4†). Among them, ADH1, ALDH7A1 and ALDH2 were also involved in glycolysis/gluconeogenesis pathway, which is closely interrelated with hepatic lipid metabolism. These findings suggest that the anti-NAFLD activity of dioscin is associated with the regulation of fatty acid metabolism pathway.Oxidative stress is one of the key factors in the “second hit” of the occurrence and development of NAFLD. Oxidative stress can activate a series of stress pathways including mitogen activated protein kinases (MAPKs), which has the pronounced roles in oxidative stress-induced cellular damages and NAFLD development.26–28 In addition, PI3K/Akt signaling pathway is also the most important component of insulin signal transduction pathway in hepatocytes. PI3K and serine–threonine protein kinase Akt can make immune cell activation by regulation of the key inflammatory cytokines and the changes in PI3K/Akt signaling pathway may lead to hepatocellular injury through activation of the mitochondrial membrane pathway of apoptosis.29–31 Our experiment showed that MAPK3 and GRB2 involved in PI3K/Akt, MAPK and insulin signaling pathways were up-regulated by dioscin compared with NAFLD group. The same result was also found in Egfr which belongs to PI3K/Akt and MAPK signaling pathways. Other protein expressions including Gng10, HSP90B1, STK4 and Fasn were also reversed by dioscin compared with model group (ESI Fig. S3 and S4†). Together with these results, dioscin ameliorated hepatic steatosis may be through effecting insulin, PI3K/Akt and MAPK signaling pathways.
PPARs is one part of nuclear receptor super-family which play a key role in modulating hepatic triglyceride accumulation in liver.32,33 In our study, 9 differentially expressed proteins involved in PPAR signaling pathway were found, and four of them were validated. Among them, ACSL1 and ACSL5 were down-regulated, while Fabpl and SLC27A2 were up-regulated, which were all reversed by dioscin (ESI Fig. S3 and S4†). These results show that dioscin may regulate fatty acid metabolism through affecting PPAR signaling pathway to play a role on resisting NAFLD.
Recent studies have found that bile acids (BA) can act as important molecules in glucose and lipid metabolic balance. Dysregulation of BA transport and impaired BA receptor signaling may contribute to the pathogenesis of NAFLD.34,35 Our study showed that BAAT, AMACR and Atp1a3 were involved in the process of bile secretion. In NAFLD liver, the expression of BAAT was increased, while the expressions of AMACR and Atp1a3 were decreased compared with normal rat. Dioscin significantly decreased the expression of BAAT, and elevated the expressions of AMACR and Atp1a3 (ESI Fig. S3 and S4†), which suggest that the anti-NAFLD activity of the compound may be associated with the regulation of bile secretion and primary bile acid biosynthesis.
Experimental section
Chemicals and reagents
Dioscin with the purity of >98% was obtained from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Silibinin was purchased from Sigma Chemical Co. (Sigma Co., Milan, Italy).Diagnostic kits for detecting levels of serum biochemistry assay were obtained from Nanjing Jiancheng Institute of Biotechnology (Nanjing, China). Insulin Radioimmunoassay Kit was purchased from Puer Biotechnology Co., Ltd. (Beijing, China). Enhanced BCA Protein Assay Kit was provided by Beyotime Institute of Biotechnology (Jiangsu, China). Tissue Protein Extraction Kit and Bradford Assay Kit were purchased from Bio-Rad Laboratories Inc. (CA, USA). Tris and sodium dodecyl sulfate (SDS) were purchased from Sigma (St Louis, MO, USA). RNAiso Plus, PrimeScript® RT reagent Kit with gDNA Eraser (Perfect Real Time) and SYBR® Premix Ex Taq™ II (Tli RNaseH Plus) were supplied by TaKaRa Biotechnology Co., Ltd. (Dalian, China).
Animals and treatment
Male Wistar rats (180–200 g) were obtained from the Experimental Animal Center of Dalian Medical University (Dalian, China, Quality certificate number: SCXK (Liao) 2008-0002). All animals were housed in the controlled environment at 23 ± 2 °C under a 12 hour dark–light cycle with free access to food and water. After being acclimatized to laboratory conditions for 1 week, animal maintenance and experiments were performed in accordance with the guidelines of the Animal Care and Use Committee.The rats were randomly divided into seven groups (n = 10): group I (normal control group) and group II (dioscin control group): fed standard chow diet (SCD), group III (model group), groups IV (positive control group) and group V–VII (low, middle and high dosages of dioscin groups): given high fat diet (HFD) including 10% hog fat, 2.5% cholesterol, 1% cholate and 10% sucrose mixed in SCD. After given SCD or HFD for 4 weeks, group II (dioscin control group) was administered dioscin (in CMC-Na, 60 mg kg−1) intragastrically (i.g.); groups IV (positive control group) was given silibinin (50 mg kg−1) intragastrically (i.g.); group V–VII were administrated dioscin at the doses of 20, 40 and 60 mg per kg per day for 12 weeks.
During the 16 weeks, all animals were constantly given SCD or HFD and weighed on the first day of the experiment and once a week to obtain total body weight. At the end of the experiments, blood samples were collected just before sacrifice and serum samples were obtained by centrifugation (3000g, 10 min, 4 °C). Liver tissues were quickly excised and completely cleaned with ice-cold PBS. The right lobe of the liver was fixed in 10% formalin to prepare paraffin sections and the remaining tissues were stored at −80 °C for other assays.
Serum biochemical assays and oral glucose tolerance test (OGTT)
The activities of ALT and AST, the levels of MDA, SOD, TC, TG, FFA, HDL, LDL, blood glucose, insulin, leptin and TNF-α in serum were detected according to the kit instructions.OGTT was performed according to the reported method.36 The curve of the blood glucose level was plotted, and the area under the curve (AUC) was calculated for the quantification of the oral glucose tolerance.
Liver morphological analysis and transmission electron microscopy (TEM)
For hematoxylin–eosin (H&E) staining and histological analysis, liver tissue was fixed in 10% neutral-buffered formalin and embedded in paraffin wax. Sections of 5 μm thick were affixed to slides, deparaffinized, and stained with HE to determine liver morphologic changes. To determine hepatic lipid accumulation, frozen sections of liver (10 μm) were stained with Sudan III (Sigma, USA). Then the sections were photographed using microscope (Nikon Eclipse TE2000-U, NIKON, Japan) at 100× magnification.The pre-treatment of liver sections for TEM imaging was carried out as previously described,37 and the images were taken using a transmission electron microscope (JEM-2000EX, JEDL, Japan).
Labeling with 4-plex iTRAQ reagents
The rat liver sample collected from control, model or dioscin-treated (60 mg kg−1) group was mixed with lysis buffer (50 mM Tris–HCl, 2.5 M thiourea, 8 M urea, 4% CHAPS, 65 mM DTT) and the total protein was extracted. Insoluble debris was pellected by centrifugation at 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g for 60 min at 4 °C. The supernatant protein was quantified by the Bradford Assay kit (BioRad, Hercules, CA) using bovine serum albumin as the standard. A total of 200 μg of each sample was reduced and digested overnight at 37 °C with trypsin (mass spectrometry grade; Promega; a protein to enzyme ratio of 20
000 × g for 60 min at 4 °C. The supernatant protein was quantified by the Bradford Assay kit (BioRad, Hercules, CA) using bovine serum albumin as the standard. A total of 200 μg of each sample was reduced and digested overnight at 37 °C with trypsin (mass spectrometry grade; Promega; a protein to enzyme ratio of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, w/w). Then they were labeled with iTRAQ reagents as follows: control, iTRAQ reagent 114 and 117; model, iTRAQ reagent 115; dioscin (60 mg kg−1), iTRAQ reagent 116 with three biological replicates for each experimental group. iTRAQ labeling was performed according to the manufacturer's protocol (Applied Biosystems, CA, USA). The labeled peptides were then pooled at a 1
1, w/w). Then they were labeled with iTRAQ reagents as follows: control, iTRAQ reagent 114 and 117; model, iTRAQ reagent 115; dioscin (60 mg kg−1), iTRAQ reagent 116 with three biological replicates for each experimental group. iTRAQ labeling was performed according to the manufacturer's protocol (Applied Biosystems, CA, USA). The labeled peptides were then pooled at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio and lyophilized to powder.
1 ratio and lyophilized to powder.
Strong cation exchange chromatography separation and nano-LC-TOF-MS/MS identification
To reduce sample complexity and remove the excess iTRAQ reagent and salts, labeled peptides were fractionated by strong cation exchange chromatography (SCX). A total of 10 fractionations were collected and dried. Further separation was achieved by nano-LC-MS/MS using an Eksigent NanoLC-Ultra® binary pump system with tray cooling (AB SCIEX, Concord, Canada) coupled to a high speed Triple TOF™ 5600 mass spectrometer (AB SCIEX, Concord, Canada). Samples were first loaded onto a Nano cHiPLC Trap column (C18-CL, 3 μm particle size, 120 Å, 200 μm × 0.5 mm) from an autosampler at 5 μl min−1 for desalting. After washing with 0.1% formic acid in HPLC grade water, the system was switched into line with the Nano cHiPLC column (C18-CL, 3 μm particle size, 120 Å, 75 μm × 15 cm). The mobile phase used for this separation was 2% acetonitrile (ACN) with 0.1% formic acid (A) and 98% ACN with 0.1% formic acid (B) and eluted at a flow rate of 300 nl min−1 using the following gradient: at 3% solvent B in A (from 0 to 12 min), 3–7% solvent B in A (from 12 to 16 min), 7–25% solvent B in A (from 16 to 50 min), 25–40% solvent B in A (from 50 to 65 min), 40–90% solvent B in A (from 65 to 75 min) and at 90% solvent B in A (from 75–85 min), with a total runtime of 120 min including mobile phase equilibration.The parameters of the TripleTOF 5600 mass spectrometer were as follows: ionspray voltage floating (ISVF) 2200 V, curtain gas (CUR) 30, interface heater temperature (IHT) 160, ion source gas 1 (GS1) 13, declustering potential (DP) 80 V. All data was acquired using information-dependent acquisition (IDA) mode with Analyst TF 1.5 software (AB SCIEX, Concord, Canada). For IDA parameters, 0.25 s MS survey scan in the mass range of 350–1250 were followed by 30 MS/MS scans of 100 ms in the mass range of 100–1500. Switching criteria were set to ions greater than mass to charge ratio (m/z) 350 and smaller than m/z 1250 with charge state of 2–5 and an abundance threshold of more than 120 counts. Former target ions were excluded for 5 s. IDA rolling collision energy (CE) parameters script was used for.
Database search and iTRAQ quantification
Protein identification was carried out using ProteinPilot Software v.4.2 (AB SCIEX, Concord, Canada). A Paragon algorithm in ProteinPilot Software was used as the default search program. International Protein Index (IPI) protein rat database version 3.87 was used to search against for protein identification. Processing parameters were set to “Biological modification” and a thorough ID search effort was used. Some important settings in the paragon search algorithm in ProteinPilot were configured as follows: sample type: iTRAQ 4 plex; Cys alkylation: iodoacetamide; digestion: trypsin; instrument: TripleTOF 5600; search effort: thorough ID. In the present study, proteins with 95% or greater confidence as determined by ProteinPilot Unused scores (≥1.3) were reported, and the corresponding false discovery rate (FDR) was less than 1%. For quantitative analysis, a protein must at minimum two unique peptide matches with iTRAQ ratios.For each protein, a ratio of its quantity in NAFLD model group and control group liver tissue (115![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 114) was obtained. The definition of a differential protein required passing these criteria: (1) the protein should meet the expression threshold of 1.5 and 0.67 (reciprocal of 1.5) for over-expressed and under-expressed proteins respectively; (2) changes had to be statistically significant (p < 0.01); (3) they had to be overlapped between two biological replicates. Proteins that meet the requirements were maintained as seed for the following bioinformatics analysis.
114) was obtained. The definition of a differential protein required passing these criteria: (1) the protein should meet the expression threshold of 1.5 and 0.67 (reciprocal of 1.5) for over-expressed and under-expressed proteins respectively; (2) changes had to be statistically significant (p < 0.01); (3) they had to be overlapped between two biological replicates. Proteins that meet the requirements were maintained as seed for the following bioinformatics analysis.
Bioinformatics analysis of proteomic data
Differentially expressed proteins were classified according to annotations from the UniProt knowledge base (Swiss-prot/TrEMBL, http://www.uniprot.org/), and the Gene Ontology (GO) database (http://david.abcc.ncifcrf.gov/) was used to elucidate biological process (BP), molecular function (MF) and cellular components (CC). Pathways were elucidated according to Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway (www.genome.ad.jp/kegg/pathway.html) associated with each differentially expressed protein. Identified proteins were further analyzed using Ingenuity Pathways Analysis (IPA) software (http://www.ingenuity.com). IPA is used to functionally analyze large datasets and identify or generate putative protein interaction clusters on the basis of a regularly updated “Ingenuity Pathways Knowledge Base” consisting of relationships between proteins, collated from annotation of the literature.Validation tests
Some differentially expressed proteins including ACADS, ACSL5, ACADVL, ALDH2, ALDH7A1, BAAT, UQCRFS1, SLC27A2, HSP90B1, MAPK3, STK4, AMACR, GRB2, CYP4A2 and ADH1 (listed in ESI Table S4†) were further verified by western blot. To eliminate the variations due to protein quantity and quality, the data were adjusted to GAPDH expression (IOD of objective protein versus IOD of GAPDH protein), and expressed as a percentage of control.In real-time PCR assay, some differentially expressed genes including Na+/K+ transporting alpha 3 polypeptide (Atp1a3), epidermal growth factor receptor (Egfr), fatty acid binding protein 1 (Fabpl), fatty acid synthase (Fasn), enoyl–CoA delta isomerase 1 (Eci1), cytochrome c oxidase, subunit VIc (Cox6c) and guanine nucleotide binding protein (G protein) gamma 10 (Gng10) were tested. Total RNA samples from liver tissue were extracted by RNAiso Plus reagent. The extracted RNA was solubilized in diethyl pyrocarbonate (DEPC)-treated RNase-free water and the amount of RNA was measured spectrophotome-trically. Only those samples with OD260/OD280 ratios between 1.8 and 2 were analyzed further. The sample concentration was calculated using the following equation: c[μg μl−1] = (OD260 − OD320) × dilution factor × 0.04. One microgram total RNA from each sample was reverse transcribed into cDNA using PrimeScript® RT reagent Kit with a TC-512 PCR system (TECHNE, UK). Real-time PCR for quantification the levels of mRNA expression was performed on a 7500 real-time PCR System (Applied Biosystems, CA). RNAiso Plus, PrimeScript® RT reagent Kit with gDNA Eraser (Perfect Real Time) and SYBR® Premix Ex Taq™ II (Tli RNaseH Plus) were supplied by TaKaRa Biotechnology Co., Ltd. (Dalian, China). The primer sequences used in real-time PCR assay are shown in Table 1. A no-template control was analyzed in parallel for each gene, and Gapdh gene was selected as the housekeeping gene in our study. The sample of each gene extracted from control group was set to 1-fold and used to determine the relative values of other samples (n-fold).
| Whole name of the genes | Abbreviations | GenBank accession | Sequences |
|---|---|---|---|
| a Automatically controlling the CE. | |||
| Na+/K+ transporting, alpha 3 polypeptide | Atp1a3 | NM_012506.1 | Forward, 5′-ATTGTGGCCAATGTCCCAGAG-3′ |
| Reverse, 5′-CAGATGGTGGATGTGGAGCCTA-3′ | |||
| Fatty acid binding protein 1, liver | Fabp1 | NM_012556.2 | Forward, 5′-GAAACCTCATTGCCACCATGAAC-3′ |
| Reverse, 5′-CTTCATGCACGATTTCTGACACC-3′ | |||
| Fatty acid synthase | Fasn | NM_017332.1 | Forward, 5′-GCTGCTACAAACAGGACCATCAC-3′ |
| Reverse, 5′-TCTTGCTGGCCTCCACTGAC-3′ | |||
| Enoyl–CoA delta isomerase 1 | Eci1 | NM_017306.4 | Forward, 5′-ATCCGAGGTGTCATCCTCACTTC-3′ |
| Reverse, 5′-GTTCCGGCCATACATCTCCATC-3′ | |||
| Cytochrome c oxidase, subunit VIc | Cox6c | NM_019360.2 | Forward, 5′-CCTAGGAGTTGCTGCTGCCTATAA-3′ |
| Reverse, 5′-CATTCTGAAATCACTTCGCACTCTG-3′ | |||
| Guanine nucleotide binding protein (G protein), gamma 10 | Gng10 | NM_053660.2 | Forward, 5′-CCAGGTCCTGTGCTTTACTTTGA-3′ |
| Reverse, 5′-CTAGGACCAGGCCACAGAGA-3′ | |||
| Epidermal growth factor receptor | Egfr | NM_031507.1 | Forward, 5′-TGCCCACCACTCATGCTGTA-3′ |
| Reverse, 5′-GCCGTGATCTGTCACCACGTA-3′ | |||
| Glyceraldehyde phosphate dehydrogenase | Gapdh | NM_017008.3 | Forward, 5′-GGCACAGTCAAGGCTGAGAATG-3′ |
| Reverse, 5′-ATGGTGGTGAAGACGCCAGTA-3′ | |||
Statistical analysis
All the data were analyzed using statistical software SPSS (version 20.0). Data are shown as mean ± standard deviation (SD). Statistical analysis of the quantitative for Multiple group comparisons was performed using the one-way analysis of variance (ANOVA) followed by Duncan's test, while couple comparisons were performed using the t-test. P-values less than 0.05 were considered to be statistically significant.Conclusions
In summary, the present study confirmed that dioscin has excellent activities against NAFLD, which were related to reduce blood lipids and blood glucose, reduce insulin resistance, relieve oxidative stress and adjust the expressions of adipokines. Furthermore, the proteomics study provided the most comprehensive proteome profile and a list of differentially expressed proteins involved in NAFLD, which should be considered as the novel markers to reveal the mechanisms and treatment of NAFLD. Bioinformatics and biological assay of these altered proteins expanded our understanding of NAFLD pathogenesis, and provide new insights for treatment of NAFLD through affecting multiple drug targets and the cross-talk of many signal pathways.Acknowledgements
This work was supported by the Doctorate in Higher Education Institutions of Ministry of Education (No. 20122105110004), the Program for Liaoning Innovative Research Team in University (LT2013019) and the Program for New Century Excellent Talents in University (NCET-11-1007).Notes and references
- E. Fassio, E. Alvarez, N. Dominguez, G. Landeira and C. Longo, Hepatology, 2004, 40, 820–826 Search PubMed.
- C. P. Day and O. F. James, Gastroenterology, 1998, 114, 842–845 Search PubMed.
- F. Elisa, S. Shelby and K. Samuel, Hepatology, 2010, 51, 679–689 CrossRef PubMed.
- M. Pérez-Carreras, P. Del Hoyo, M. A. Martín, J. C. Rubio, A. Martín and G. Castellano, Hepatology, 2003, 38, 999–1007 CrossRef PubMed.
- D. G. Tiniakos, M. B. Vos and E. M. Brunt, Annu. Rev. Pathol.: Mech. Dis., 2010, 5, 145–171 CrossRef CAS PubMed.
- N. E. Sunny, E. J. Parks, J. D. Browning and S. C. Burgess, Cell Metab., 2011, 14, 804–810 Search PubMed.
- E. R. Kallwitz, A. McLachlan and S. J. Cotler, World J. Gastroenterol., 2008, 14, 22–28 CrossRef CAS.
- S. Matsuda, M. Kobayashi and Y. Kitagishi, ISRN Endocrinol., 2013, 2013, 472432 Search PubMed.
- J. Wanninger, M. Neumeier, J. Weigert, S. Bauer, T. S. Weiss and A. Schäffler, Am. J. Physiol., 2009, 297, G611–618 CrossRef CAS PubMed.
- V. S. Calvert, R. Collantes, H. Elariny, A. Afendy, A. Baranova and M. Mendoza, Hepatology, 2007, 46, 166–172 Search PubMed.
- X. Zhang, J. Yang, Y. Guo, H. Ye, C. Yu and C. Xu, Hepatology, 2010, 51, 1190–1199 CrossRef CAS PubMed.
- M. Blueggel, D. Chamrad and H. E. Meyer, Curr. Pharm. Biotechnol., 2004, 5, 79–88 CAS.
- E. Rodríguez-Suárez, A. M. Duce, J. Caballería, F. Martínez Arrieta, E. Fernández and C. Gómara, Proteomics: Clin. Appl., 2010, 4, 362–371 CrossRef PubMed.
- L. N. Bell, J. L. Theodorakis, R. Vuppalanchi, R. Saxena, K. G. Bemis and M. Wang, Hepatology, 2010, 51, 111–120 Search PubMed.
- C. Martel, D. D. Esposti, A. Bouchet, C. Brenner and A. Lemoine, Curr. Pharm. Biotechnol., 2012, 13, 726–735 CAS.
- D. S. Svoboda and M. D. Kawaja, J. Proteomics, 2012, 75, 1752–1763 CrossRef CAS PubMed.
- E. Rodriguez-Suarez, J. M. Mato and F. Elortza, Methods Mol. Biol., 2012, 909, 241–258 CAS.
- H. Li, W. Huang, Y. Q. Wen, G. H. Gong, Q. B. Zhaoand and G. Yu, Fitoterapia, 2010, 81, 1147–1156 CrossRef CAS PubMed.
- M. Takechi, S. Shimada and Y. Tanaka, Phytochemistry, 1991, 30, 3943–3944 CrossRef CAS.
- T. Ikeda, J. Ando, A. Miyazono, X. H. Zhu, H. Tsumagari and T. Nohara, Biol. Pharm. Bull., 2000, 23, 363–364 CAS.
- M. M. Hu, L. N. Xu, L. H. Yin, Y. Qi, H. Li and Y. W. Xu, J. Appl. Toxicol., 2013, 33, 712–722 CrossRef CAS PubMed.
- B. N. Lu, L. H. Yin, L. N. Xu and J. Y. Peng, Planta Med., 2011, 77, 407–415 CrossRef CAS PubMed.
- B. N. Lu, Y. W. Xu, L. N. Xu, X. N. Cong, L. H. Yin and H. Li, Environ. Toxicol. Pharmacol., 2012, 34, 127–135 CrossRef CAS PubMed.
- X. M. Zhao, X. N. Cong, L. L. Zheng, L. N. Xu, L. H. Yin and J. Y. Peng, Toxicol. Lett., 2012, 214, 69–80 Search PubMed.
- M. Kohjima, M. Enjoji, N. Higuchi, M. Kato, K. Kotoh and T. Yoshimoto, Int. J. Mol. Med., 2007, 20, 351–358 CAS.
- H. Kojima, S. Sakurai, M. Uemura, H. Fukui, H. Morimoto and Y. Tamagawa, Alcohol.: Clin. Exp. Res., 2007, 31, S61–S66 Search PubMed.
- I. A. Leclercq, G. C. Farrell, J. Field, D. R. Bell, F. J. Gonzalez and G. R. Robertson, J. Clin. Invest., 2000, 105, 1067–1075 CrossRef CAS PubMed.
- S. Aghazadeh and R. Yazdanparast, Clin. Nutr., 2010, 29, 381–385 Search PubMed.
- F. Zhang, Z. Zhang, D. Kong, X. Zhang, L. Chen and X. Zhu, Mol. Cell. Endocrinol., 2013, 382, 197–204 Search PubMed.
- J. W. Han, X. R. Zhan, X. Y. Li, B. Xia, Y. Y. Wang and J. Zhang, World J. Gastroenterol., 2010, 16, 6111–6118 CrossRef CAS.
- K. Cusi, K. Maezono, A. Oaman, M. Pendergrass, M. E. Patti and T. Pratipanawatr, J. Clin. Invest., 2000, 105, 311–320 CrossRef CAS PubMed.
- T. Aoyama, J. M. Peters, N. Iritani, T. Nakajima, K. Furihata and T. Hashimoto, J. Biol. Chem., 1998, 273, 5678–5684 CrossRef CAS PubMed.
- C. Dreyer, G. Krey, H. Keller, F. Givel, G. Helftenbein and W. Wahli, Cell, 1992, 68, 879–887 CrossRef CAS.
- P. B. Hylemon, H. Zhou, W. M. Pandak, S. Ren, G. Gil and P. Dent, J. Lipid Res., 2009, 50, 1509–1520 CrossRef CAS PubMed.
- M. Trauner, T. Claudel, P. Fickert, T. Moustafa and M. Wagner, Dig. Dis., 2010, 28, 220–224 CrossRef PubMed.
- Y. Q. Li, H. Ji, Y. H. Zhang, W. B. Shi, Z. K. Meng and X. Y. Chen, Eur. J. Pharmacol., 2007, 577, 100–108 CrossRef CAS PubMed.
- J. A. Ibdah, P. Perlegas, Y. Zhao, J. Angdisen, H. Borgerink and M. K. Shadoan, Gastroenterology, 2005, 128, 1381–1390 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra03948c |
| This journal is © The Royal Society of Chemistry 2014 |