Vanillic Mannich bases: synthesis and screening of biological activity. Mechanistic insight into the reaction with 4-chloroaniline†
Abstract
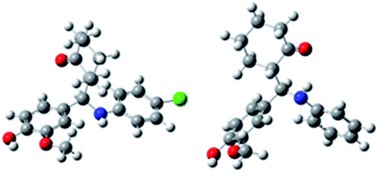
One-step multi-component Mannich reaction of vanillin, aromatic amines (aniline and 4-chloroaniline), and cyclohexanone was successfully catalyzed by three chloroacetate ethanolamine based ionic liquids: diethanolammonium chloroacetate, and newly synthesized ethanolammoniumchloroacetate and N,N-diethylethanolammoniumchloroacetate. These reactions were performed in ethanol at room temperature. Mechanistic aspects of the reaction with 4-chloroaniline were considered by using density functional theory. The yield of obtained Mannich bases (MB-Cl and newly synthesized MB-H) was very good, while diastereoselectivity was excellent. These compounds were evaluated for their in vitro antioxidative activity by DPPH free radical scavenging assay. It was shown that both bases exhibit high activity against DPPH. In vitro cytotoxic and antioxidative effects of MB-Cl and MB-H against human breast carcinoma MDA-MB-231 and human colon carcinoma HCT-116 cell lines were also determined. The investigated Mannich bases show moderate or very weak cytotoxic effect on HCT-116 cells, while no cytotoxic effect was observed in the case of MDA-MB-231 cells. On the other hand, the tested substances induced oxidative stress in the treated cancer cell lines.

 Please wait while we load your content...
Please wait while we load your content...