Borontribromide-mediated C–C bond formation in cyclic ketones: a transition metal free approach†
Abstract
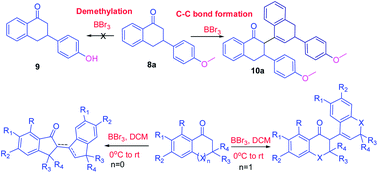
Borontribromide (BBr3) is a well-known demethylating agent. The current investigation was focused on a new application of borontribromide as a C–C bond forming agent in cyclic ketones. In this study, borontribromide mediated C–C bond formation reactions of tetralones, chromenone, thiochromenone and indanones were explored. A methoxy group containing ketones showed selective C–C bond formation reaction instead of demethylation of the methoxy group. MM2 steric energy calculations for the final products showed that the reaction favored the formation of exo- or endo-cyclic double bond containing products, depending upon their low MM2 steric energy in a specific frame structure, as observed in X-ray crystallography. A comprehensive crystallographic and pi-stacking analysis of product 10a demonstrated the formation of 10a as an enantiomeric mixture, and its centre of inversion was stabilized by a set of three unique pi–pi interactions.

 Please wait while we load your content...
Please wait while we load your content...