Synthesis, biological evaluation, and molecular docking studies of novel chalcone oxime derivatives as potential tubulin polymerization inhibitors†
Yan-Ting Wang‡
a,
Ya-Juan Qin‡a,
Ya-Liang Zhanga,
Yu-Jing Lia,
Bing Raoa,
Yan-Qing Zhanga,
Meng-Ru Yanga,
Ai-Qin Jiang*b,
Jin-Liang Qi*a and
Hai-Liang Zhu*a
aState Key Laboratory of Pharmaceutical Biotechnology, School of Life Sciences, Nanjing University, Nanjing 210093, P. R. China. E-mail: zhuhl@nju.edu.cn; Fax: +86-25-8359 2672; Tel: +86-25-8359 2572
bSchool of Medicine, Nanjing University, Nanjing 210093, P. R. China
First published on 2nd July 2014
Abstract
Microtubule-targeted drugs are at present indispensable for various types of cancer therapy worldwide. A series of chalcone oxime derivatives were designed, synthesized and evaluated as potential tubulin polymerization inhibitors and for the cytotoxicity against anthropic cancer cell lines. These derivatives were completely demonstrated to have commendable inhibitory activity against tubulin polymerization by competing with the colchicine-binding site on tubulin, which was associated with G2/M phase cell cycle arrest as well as promising antiproliferative activity. Among the novel compounds, compound 13 was demonstrated to have the most potent inhibitory activity (tubulin IC50 = 1.6 μM). Antiproliferative assay results displayed that compound 13 had potent antiproliferative activity against A549, Hela and MCF-7 with GI50 values of 2.1, 3.5 and 3.6 μM, respectively, which were compared with the positive control colchicine and CA-4. Docking simulation showed that compound 13 could bind tightly to the colchicine domain of tubulin and act as a tubulin polymerization inhibitor. We also built a 3D-QSAR model to provide more information that could be applied to design new molecules with more potent tubulin inhibitory activity.
1. Introduction
Microtubules are structural components of the mitotic spindle, and dynamic microtubules play an important role in several cellular processes including intracellular trafficking, cell migration and cell division.1 Tubulin, the major protein component of microtubules, is the target of numerous antimitotic drugs.2–4 Several mitotic inhibitors are known to inhibit microtubule assembly5–9 and the inhibition of microtubule assembly dynamics has been shown to be the mode of action for several clinically successful anticancer drugs. Colchicine (Fig. 1) is the first drug that is well known to bind to the tubulin, and its binding site on tubulin has been characterized already.10 Besides, vinblastine, vincristine, estramustine, combretastatin A-4 and paclitaxel are now also used as anticancer agents clinically.8,9 In addition to their clinical applications in diverse types of diseases including cancer, fungal and parasitic diseases,11 microtubule inhibitors are also highly useful for understanding the role of microtubules in the cellular processes.12,13 Microtubule inhibitors generally block cell cycle progression in mitosis and a prolonged mitotic-arrest triggers various apoptotic pathways.9,14,15Among the naturally occurring compounds, combretastatin A-4 (Fig. 1), isolated from the bark of the South African tree Combretum caffrum,16 is a highly effective natural tubulin-binding molecule affecting microtubule dynamics by binding to the colchicine site.17 Several other new compounds have also been synthesized as antitubulin agents, such as MDL-27048, BNC 105 and chalcone (Fig. 1). They bind to tubulin with the same site as colchicine.18 Among these structures, it is widely reported that chalcone is a promising skeleton that has shown good antitubulin activity. It is abundant in edible plants and displays a variety of biological activities, such as anti-cancer,19–21 anti-fungal,22 anti-tuberculosis,23 and anti-inflammatory activities.24,25 Its broad biological properties are largely due to the α,β-unsaturated ketone moiety. And there are a great quantity of evidences, derived from molecular modeling studies and experimental data, demonstrating how this class of inhibitors bind to the active site of tubulin.26–29 Therefore, in order to develop more potent tubulin inhibitors as antitumor agent, we attempt to use the chalcone as the basic scaffold for the design of a series of novel tubulin polymerization inhibitors. Some substituents was introducted onto the two aromatic rings, and the modifications have been performed to screen active chalcones,30 such as MDL-27048 (Fig. 1).31
To further optimize the potency of chalcone analogues and to gain further insight in their structure–activity relationships (SARs), we are now searching more novel agents to inhibit tubulin. Oxime formation was used as bond which could attach chemotherapeutic agents to targeting moiety in previous reports.32 Besides, the biological relevance of oximes appreciably favors their use as ligands for potential metal-based drugs, which may yield increased cytotoxicity by virtue of synergistic effects between the platinum center and the coordinated oxime.33
Here oxime was introduced into chalcone obtaining a series of novel chalcone oxime in which oxime served as a primary structure. Then different substituents have been introduced onto the two aromatic rings. Our group previously do some research about antitubulin agents,23,34 and the structures of novel chalcone oxime are similar with the agents. So the work in this paper is a continuation of them.
The docking study based on tubulin crystal structure (PDB code: 1SA0) indicated compounds 10 and 13 exhibit more affinity for tubulin than colchicine, which rationally proved the reason why these compounds also possess effective inhibitory activity profile against tubulin.
In this paper, some research has been done: discuss the synthetic method of this series of compounds, depict the results of reactivity studies; evaluate their anticancer and antitubulin activities; research the influence on the cell division cycle and cell apoptosis and analysis the structure–activity relationship. Additionally, molecular docking and 3D-QSAR model provided more information that could be applied to design new molecules with more potent tubulin inhibitory activity.35
2. Results and discussion
2.1. Chemistry
The synthetic route for the novel chalcone oxime derivatives 7–24 is outlined in Scheme 1. These compounds were synthesized from chalcone 1a–7h and hydroxylamine hydrochloride. Compounds 1a–7h were prepared according to the procedure reported by Dalmolen et al. with some modifications.36 Substituted benzaldehyde and substituted acetophenone were dissolved in cool ethyl alcohol, and sodium hydroxide was added dropwise. The reaction mixture was then stirred at room temperature for 16 h, and chalcones were obtained with yields of 86–91%. Then, chalcone 1a–7h and hydroxylamine hydrochloride were dissolved in ethyl acetate, and refluxed to give the desired compounds 7–24 (Table 1).| Compounds | R1 | R2 | R3 | R4 | R5 | R6 | R7 |
|---|---|---|---|---|---|---|---|
| 7 | H | –OCH3 | –OCH3 | H | H | H | –OCH3 |
| 8 | –OCH3 | H | –OCH3 | –OCH3 | H | H | –OCH3 |
| 9 | –OCH3 | H | –OCH3 | –OCH3 | H | H | –OC2H5 |
| 10 | –OCH3 | H | –OCH3 | H | –OCH3 | –OCH3 | H |
| 11 | –OCH3 | H | –OCH3 | H | –OCH3 | Br | H |
| 12 | –OCH3 | H | –OCH3 | H | –OCH3 | Cl | Cl |
| 13 | –OCH3 | H | –OCH3 | H | –OCH3 | NH2 | H |
| 14 | –OCH3 | H | –OCH3 | H | –OCH3 | NO2 | H |
| 15 | –OCH3 | H | –OCH3 | H | –OCH3 | H | Br |
| 16 | H | –OCH3 | –OCH3 | –OCH3 | H | H | –OC2H5 |
| 17 | H | H | –OCH3 | H | H | H | –OC2H5 |
| 18 | H | H | –OCH3 | H | H | Cl | Cl |
| 19 | –OCH3 | H | H | H | H | H | –OC2H5 |
| 20 | H | –OCH3 | H | H | H | H | –OCH3 |
| 21 | –OCH3 | H | –OCH3 | H | –OCH3 | H | H |
| 22 | –OCH3 | H | –OCH3 | H | –OCH3 | H | F |
| 23 | –OCH3 | H | –OCH3 | H | –OCH3 | H | –OCH3 |
| 24 | –OCH3 | H | –OCH3 | –OCH3 | H | Cl | Cl |
2.2. Crystal structure determination
Among them, a crystal structure of compound 17, 19 and 24 were determined by X-ray diffraction analysis. The crystal data presented in Table 2 and Fig. 2 give perspective views of 17, 19 and 24 in the form of trans-isomerism with the atomic labeling system.| Compounds | 17 | 19 | 24 |
|---|---|---|---|
| Formula | C18H19NO3 | C18H19NO3 | C18H17Cl2NO4 |
| Formula weight | 297.35 | 297.35 | 343.37 |
| Crystal system | Monoclinic | Monoclinic | Monoclinic |
| Space group | P21/n | P21/n | P21/n |
| a (Å) | 10.2117(11) | 5.8357(10) | 5.7733(9) |
| b (Å) | 12.3644(11) | 9.4747(16) | 12.2808(19) |
| c (Å) | 12.878(11) | 14.918(3) | 12.979(2) |
| α (o) | 93.100(3) | 72.258(5) | 90.460(4) |
| β (o) | 96.848(3) | 81.924(6) | 94.633(4) |
| γ (o) | 90.294(3) | 89.067(5) | 102.396(4) |
| V (Å3) | 1611.9(3) | 777.5(2) | 895.5(2) |
| Z | 32 | 16 | 12 |
| Dc (g cm−3) | 1.418 | 1.470 | 1.746 |
| μ (mm−1) | 0.127 | 0.132 | 0.995 |
| F(000) | 704 | 352 | 468 |
| θrang (o) | 2.23–25.07 | 2.26–25.07 | 2.29–25.08 |
| Reflns collected | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 443 443 |
6917 | 7718 |
| Reflns unique | 5704 | 2735 | 3178 |
| Goodness-of-fit on F2 | 1.008 | 1.537 | 1.002 |
| R1, wR2[I > 2σ(I)] | 0.0502, 0.1038 | 0.1252, 0.3787 | 0.0707, 0.1122 |
| R1, wR2[all data] | 0.1157, 0.1254 | 0.1703, 0.4085 | 0.1765, 0.1409 |
| Max, min Δρ (e Å−3) | 0.161, −0.174 | 1.606, −0.523 | 0.261, −0.233 |
 | ||
| Fig. 2 (A) Crystal structure diagram of compound 17. (B) Crystal structure diagram of compound 19. (C) Crystal structure diagram of compound 24. H atoms are shown as small spheres of arbitrary radii. | ||
2.3. Biological evaluation
| Compounds | GI50 ± SD (μM) | Tubulin assemblyb IC50 ± SD (μM) | Inhibition of colchifine bindingc (% ± SD) | ||
|---|---|---|---|---|---|
| A549a | Helaa | MCF-7a | |||
| a A549 (human lung adenocarcinoma cells), Hela (human cervical carcinoma cells) and MCF-7 (human breast carcinoma cells). Cancer cells were purchased from NanJing KeyGen Biotech Co., Ltd., which subcultured by State Key Laboratory of Pharmaceutical Biotechnology.b Inhibition of tubulin polymerization.c Inhibition of colchifine binding.d Used as a positive controls. | |||||
| 7 | 67.6 ± 3.4 | 65.7 ± 0.22 | 77.2 ± 3.4 | 8.5 ± 1.4 | 57 ± 0.3 |
| 8 | 18.6 ± 4.7 | 18.1 ± 0.23 | 22.2 ± 0.55 | 3.8 ± 0.43 | 82 ± 2.6 |
| 9 | 6.9 ± 8.2 | 11.2 ± 0.45 | 18.7 ± 0.32 | 2.9 ± 1.2 | 83 ± 0.3 |
| 10 | 2.8 ± 9.4 | 6.6 ± 0.21 | 5.8 ± 0.06 | 1.9 ± 0.34 | 92 ± 0.4 |
| 11 | 29.6 ± 5.4 | 34.3 ± 0.56 | 41.2 ± 0.32 | 3.6 ± 0.44 | 70 ± 0.3 |
| 12 | 32.5 ± 3.2 | 37.3 ± 0.05 | 43.3 ± 0.56 | 4.1 ± 0.62 | 65 ± 2.6 |
| 13 | 2.1 ± 5.1 | 3.5 ± 0.23 | 3.6 ± 0.31 | 1.6 ± 0.32 | 95 ± 0.2 |
| 14 | 41.4 ± 3.9 | 52.1 ± 0.42 | 57.7 ± 0.32 | 4.9 ± 0.65 | 63 ± 0.6 |
| 15 | 45.3 ± 3.3 | 54.9 ± 0.04 | 67.8 ± 0.76 | 5.2 ± 0.76 | 62 ± 0.8 |
| 16 | 4.6 ± 6.3 | 8.9 ± 0.32 | 17.4 ± 0.04 | 2.6 ± 0.54 | 85 ± 4.3 |
| 17 | 73.5 ± 7.6 | 75.4 ± 0.62 | 83.3 ± 5.7 | 11.4 ± 2.3 | 55 ± 0.6 |
| 18 | 112 ± 3.2 | 132 ± 4.4 | 121 ± 9.7 | 46.3 ± 5.7 | 51 ± 1.4 |
| 19 | 91.8 ± 7.2 | 82.9 ± 0.33 | 97.2 ± 12.1 | 13.5 ± 3.5 | 53 ± 2.9 |
| 20 | 104 ± 8.1 | 113 ± 1.3 | 105 ± 2.4 | 38.4 ± 4.5 | 51 ± 0.9 |
| 21 | 25.3 ± 4.4 | 28.3 ± 0.08 | 34.6 ± 0.43 | 3.4 ± 0.56 | 72 ± 0.2 |
| 22 | 62.3 ± 0.55 | 59.8 ± 0.08 | 74.5 ± 0.66 | 6.3 ± 0.97 | 59 ± 0.6 |
| 23 | 3.2 ± 1.2 | 7.7 ± 0.20 | 8.9 ± 0.02 | 2.2 ± 0.44 | 87 ± 1.6 |
| 24 | 19.4 ± 3.4 | 22.3 ± 0.43 | 27.6 ± 0.21 | 3.3 ± 0.52 | 80 ± 0.1 |
| Colchicined | 10.4 ± 3.8 | 13.2 ± 2.3 | 17.5 ± 5.7 | 1.92 ± 4.8 | — |
| CA-4d | 3 ± 0.04 | 9.3 ± 0.01 | 8.4 ± 0.04 | 1.80 ± 0.20 | 93% ± 0.4 |
Structure–activity relationships in these chalcone oxime derivatives demonstrated as follows:
Analysis of substituent groups in the A ring: compounds 17 with p-substituted group showed slightly more potent activities than those of o-substituted 19. Antiproliferative activity of compounds 9, 7 and 17, compounds 12, 24 and 18, compounds 23, 8, and 20 was reducing gradually respectively, which can demonstrate that the more substituted groups in the A ring, the better antiproliferative activity it was. Furthermore, antiproliferative activity of compound 16 was better than 9; 23 was better than 8, which can demonstrate that 2,4,6-trimethoxy group in A ring was better than 3,4,5-trimethoxy group. Antiproliferative activity of compound 12 with 2,4,6-methoxy group in A ring was better than compound 24 with 2,4,5-methoxy group in A ring.
Analysis of substituent groups in the B ring: the compounds with meta-substituted 10 and 11 exhibited slightly better antiproliferative activity than para substituted compounds separately 23 and 15. The compounds 10, 13 and 23 with electron donating group exhibited more significant antiproliferative activities than 21, which had positive effects relative to those electron-withdrawing substituted of 11, 12, 14, 15 and 22. This results demonstrated that an electron donating group in B ring may have slightly improved antiproliferative activity. Compound 17 owing more significant antiproliferative activities than 18 also demonstrated a better antiproliferative activities of electron donating group than electron-withdrawing group.
Strikingly, the most potent small molecule screened via these antiproliferative assays was compound 13 which suppresses the A549, Hela, and MCF-7 cells with the GI50 = 2.1 μM and GI50 = 3.5 μM, GI50 = 3.6 μM respectively.
The new chalcone oximes were also examined for potential inhibition of the binding of [3H] colchicine to tubulin (Table 3). Compound 13 showed the best inhibition of the binding reaction (95% inhibition), and was better than CA-4 (93% inhibition). Of all the new compounds, the most potent were also compounds 10 and 13. In this assay, it was observed that compound 13 was the strongest inhibitor.
In the vehicle treated group, about 0.04% of A549 cells were distributed in the G2/M phase. Compound 13 increased the proportion of cells in G2/M phase in a concentration-dependent manner (Fig. 3). About 16% of cells were found in G2/M phase when treated with 0.1 μM compound 13 for 24 h; about 34% of cells for 0.3 μM; and about 40% of cells for 0.5 μM.
2.4. Docking simulations
To gain better understanding on the potency of the synthesized compounds and guide further SAR studies, we proceeded to examine the interaction of the chalcone oxime derivatives with tubulin crystal structure (PDB code: 1SA0).Data was provided for the molecular docking simulations for the synthesized compounds 7–24 in Table 5. The predicted binding interaction energy (kcal mol−1) was used as the criterion for ranking. The molecular docking was performed by inserting the compound into the colchicine binding site of tubulin. All docking runs were applied by Discovery Studio 3.5. As depicted in Table 5, compound 13 showed the biggest interaction energy, which means that compound 13 exhibited the most potent affinity for tubulin in prediction. And the binding models of compound 13 and tubulin were depicted in Fig. 5. The amino acid residues which had interaction with tubulin were labeled.
The obtained results were presented in the two groups of pictures. Fig. 5 showed the binding mode of compound 13 interacting with 1SA0 protein and the docking results revealed that three amino acids Thr179, Cys241 and Lys352 located in the binding pocket of protein played vital roles in the conformation with compound 13, which were stabilized by one Pi-cation bond, and two hydrogen bonds that were shown in 2D diagram. The Pi-cation bond with 5.3 Å was formed between amino acid Lys352 and benzene ring B of compound 13; one hydrogen bond was formed also relating to Lys352, which connected to hydrogen atom of oxime part with 2.2 Å; the second hydrogen bond with 2 Å was formed between Thr179 and hydrogen atom of amidogen; and the last hydrogen bond with 2.4 Å was involved in Cys241 and the oxygen atom of methoxyphenol on benzene ring A.
This molecular docking result in a molecular level foundation, along with the biological assay data, suggested that compounds 13 was the most potential inhibitors of tubulin.
2.5. QSAR model
To evaluate the synthesized compounds as tubulin inhibitors systematically and explore more potent inhibitors, eighteen compounds with definite IC50 values against tubulin were selected as the model dataset by using the Create 3D QSAR protocol of Discovery Studio 3.5. 3D-QSAR model was built by using the corresponding pIC50 values which were converted from the obtained IC50 (μM) values of tubulin inhibition and performed by built-in QSAR software of DS 3.5 (Discovery Studio 3.5, Accelrys, Co. Ltd). The way of this transformation was derived from an online calculator developed from an Indian medicinal chemistry lab (http://www.sanjeevslab.org/tools-IC50.html).37 The training and test set were chosen by the Diverse Molecules method in Discovery Studio. Considering a good alignment is very important for the analysis of molecular fields, we applied CDOCKER protocol to explore each molecule with lowest energy before alignment conformation. Chalcone oxime was selected as substructure to build alignment conformation before building the QSAR model.The training and test set were divided by the random diverse molecules method of DS 3.5, in which the test set accounts for 77.8% while the training set was set to 22.2%. The training set composes 14 agents and 4 agents were consisted of the relative test set, which had been presented in Table 4. The main purpose to create 3D-QSAR model was to choose activity conformation of the designed molecular and reasonably evaluated the designed molecules. The success of this model depended on docking study and the reliability of previous study about activities data.38
| Compounds | Tubulin assemblya | Residual error | |
|---|---|---|---|
| Actual pIC50 | Predicted pIC50 | ||
| a Italicised compounds were selected as the test sets, while the rest ones were in the training sets. | |||
| 7 | 5.072 | 5.016 | 0.056 |
| 8 | 5.511 | 5.534 | −0.023 |
| 9 | 5.542 | 5.156 | 0.386 |
| 10 | 5.741 | 5.779 | −0.038 |
| 11 | 5.443 | 5.419 | 0.024 |
| 12 | 5.392 | 5.408 | −0.016 |
| 13 | 5.801 | 5.827 | −0.026 |
| 14 | 5.314 | 5.450 | −0.136 |
| 15 | 5.283 | 5.284 | −0.001 |
| 16 | 5.592 | 5.584 | 0.008 |
| 17 | 4.943 | 5.002 | −0.059 |
| 18 | 4.332 | 4.361 | −0.029 |
| 19 | 4.871 | 4.814 | 0.057 |
| 20 | 4.421 | 4.464 | −0.043 |
| 21 | 5.472 | 5.390 | 0.082 |
| 22 | 5.201 | 5.241 | −0.04 |
| 23 | 5.664 | 5.617 | 0.047 |
| 24 | 5.482 | 5.451 | 0.031 |
| Compounds | Intramolecular energy for ligands (kcal mol−1) | Ligand–protein interactions (kcal mol−1) |
|---|---|---|
| 7 | −17.3 | −47.3 |
| 8 | −18.7 | −56.7 |
| 9 | −19.6 | −57.6 |
| 10 | −15.8 | −60.8 |
| 11 | −19.2 | −52.2 |
| 12 | −21.9 | −51.9 |
| 13 | −22.5 | −62.5 |
| 14 | −18.8 | −50.8 |
| 15 | −16.7 | −48.7 |
| 16 | −19.7 | −57.9 |
| 17 | −21.6 | −43.6 |
| 18 | −20.8 | −40.8 |
| 19 | −17.7 | −42.7 |
| 20 | −16.6 | −41.6 |
| 21 | −16.6 | −52.6 |
| 22 | −18.2 | −48.2 |
| 23 | −20.1 | −60.1 |
| 24 | −17.7 | −55.2 |
| Colchicine | −16.5 | −56.5 |
In default situation, the alignment conformation of each molecule was the one that possessed the lowest CDOCKER_INTERACTION_ENENGY among the eighteen docked poses. The 3D-QSAR model generated from DS 3.5, defined the critical regions (steric or electrostatic) affecting the binding affinity. It was a PLS model set up 400 independent variables (conventional R2 = 0.9343). The observed and predicted values, corresponding residual values for the training set and test set molecules in 3D-QSAR model, were presented in Table 4. The well agreement between predicted pIC50 value and experimental pIC50 value for both test sets and training sets were shown in Fig. 6.
 | ||
| Fig. 6 Using linear fitting curve to compare the predicted pIC50 value (tubulin inhibitory activities) with that of experimental pIC50. | ||
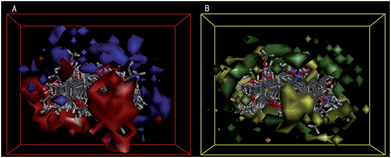
Also the molecules aligned with the iso-surfaces of the 3D-QSAR model coefficients on van der Waals grids (Fig. 7A) and electrostatic potential grids (Fig. 7B) were listed. Electrostatic map indicated red contours around regions where high electron density (negative charge) is expected to increase activity, and blue contours represented areas where low electron density (partial positive charge) is expected to increase activity. Similarly, steric map indicates areas where steric bulk is predicted to increase (green) or decrease (yellow) activity. It was widely acceptable that a better inhibitor in the 3D QSAR model should have strong van der Waals attraction in the green areas and a polar group in the blue electrostatic potential areas (which were dominant close to the skeleton). As shown in the two pictures, the potent compound 13 not only could circumvent the red subregion or the unfavorable yellow steric subregion but can also get more close to the favorable blue and green spaces. Thus, this promising model would provide a guideline to design and optimize more effective tubulin inhibitors based on the chalcone oxime analogues skeleton and pave the way for us to further study in future.
3. Conclusion
In summary, novel chalcone oxime analogues were synthesized based on rational structural modification of previous chalcone oxime analogues. Structure–activity relationships were investigated by introducing different substituents into the two aromatic rings. Several chalcone oximes showed excellent antiproliferative activity and tubulin polymerization inhibition activity. Among them, compound 13 was the most potent one, with IC50 in the low micromole range, and also provided very interesting insights in future optimization of these analogues. Mechanism of action studies confirmed that chalcone oxime analogues maintain their ability to inhibit tubulin polymerization at colchicine binding site; arresting cells in G2/M phase and inducing cell apoptosis. And the docking study based on tubulin crystal structure (PDB code: 1SA0) also indicated that compound 13 exhibited the most potent affinity for tubulin. These results strongly suggested that novel chalcone oxime analogues can be further developed as a promising antitumor agent for the more efficacious treatment of advanced cancers.4. Experimental section
4.1. Materials and measurements
All chemicals and reagents used in current study were analytical grade. Thin layer chromatography (TLC), proton nuclear magnetic resonance (1H NMR) and elemental microanalyses (CHN) were usually used. Analytical thin-layer chromatography (TLC) was performed on the glass-backed silica gel sheets (silica gel 60 Å GF254). All compounds were detected using UV light (254 nm or 365 nm). Separation of the compounds by column chromatography was carried out with silica gel 60 (200–300 mesh ASTM, E. Merck). The quantity of silica gel used was 50–100 times the weight charged on the column. Melting points were determined on a XT4 MP apparatus (Taike Corp., Beijing, China). 1H NMR spectra were measured on a Bruker AV-300 or AV-500 spectrometer at 25 °C and referenced to Me4Si. Chemical shifts are reported in ppm (δ) using the residual solvent line as internal standard. Splitting patterns are designed as s, singlet; d, doublet; t, triplet; m, multiplet. ESI-MS spectra were recorded on a Mariner System 5304 Mass spectrometer. Elemental analyses were performed on a CHN–O-Rapid instrument and were within ±0.4% of the theoretical values.4.2. General procedure for preparation of compounds 1a–7h
In a round bottom flask (100 mL), placed in an ice bath, 20 mL of EtOH, 10 mmol of acetophenone or derivatives, 11 mmol of benzaldehyde or derivatives and 15 mL of 10% NaOH solution were added. Then kept on magnetic stirring at 40 °C for several hours (monitored by TLC). After the reaction was completed, the mixture was cooled slowly and left in freezer for 48 h and then filtered under vacuum. The product was crystallized in ethanol and methanol. Chalcone 1a–7h were obtained in yields of 81–98%.4.3. General procedure for preparation of compounds 7–24
A mixture of chalcone (1 mmol), hydroxylamine hydrochloride (3 mmol), potassium carbonate, (1 mmol) and anhydrous sodium sulfate (1.5 mmol) was refluxed in ethyl acetate (15 mL) under stirring. During this period the reaction was followed by TLC on silica gel as indicated in Table 1. After the reaction completion, the mixture was filtered, and the solvent was evaporated under reduced pressure. Then distilled water (15 mL) and dichloromethane (15 mL × 3) were added to extract organic compounds. The organic extracts were combined, dried over anhydrous MgSO4, filtered and concentrated under reduced pressure. The crude mixture was obtained and purified by silica gel column chromatography (200–300 mesh) eluted with a mixture of ethyl acetate and petroleum ether (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) to afford pure products.
2) to afford pure products.
4.4. Crystal structure determination
Crystal structures determination of compound 17, 19 and 24 were carried out on a Nonius CAD4 diffractometer equipped with graphite-mono chromated MoKa (k = 0.71073 Å) radiation. The structures were solved by direct methods and refined on F2 by fullmatrix least-squares methods using SHELX-97.39 All non-hydrogen atoms of compound 17, 19 and 24 were refined with anisotropic thermal parameters. All hydrogen atoms were placed in geometrically idealized positions and constrained to ride on their parent atoms.4.5. Cell proliferation assay (cell viability was assessed by MTT assay)
We evaluated the antiproliferativities of compounds 7–24 against A549 (human lung adenocarcinoma cells), Hela (human cervical carcinoma cells), and MCF-7 (human breast carcinoma cells) with analysis. Cell proliferation was determined using MTT dye (Beyotime Inst Biotech, China) according to the instructions of manufacture. Briefly, 1–5 × 103 cells per well were seeded in a 96-well plate, grown at 37 °C for 12 h. Subsequently, cells were treated with compounds (Table 3) at increasing concentrations in the presence of 10% FBS for 24 h. After 10 μL MTT dye was added to each well, cells were incubated at 37 °C for 3–4 h. Then all the solution in the wells was poured out and 150 μL DMSO was added to every well. Plates were read in a Victor-V multilabel counter (Perkin-Elmer) using the default europium detection protocol. Percent inhibition or GI50 values of compounds were calculated by comparison with DMSO-treated control wells. The results are shown in Table 3.4.6. Effects on tubulin polymerization and on colchicine binding to tubulin
To evaluate the effect of the compounds on tubulin polymerization in vitro, different concentrations of 20 compounds (containing colchicine and CA-4) were preincubated with 10 μM bovine brain tubulin in glutamate buffer at 30 °C and then cooled to 0 °C. After addition of 0.4 mM GTP, the mixtures were transferred to 0 °C cuvettes in a recording spectrophotometer and warmed to 30 °C. Tubulin polymerization was followed turbidimetrically at 350 nm. The IC50 was defined as the compound concentration that inhibited the extent of polymerization by 50% after 20 min incubation.The capacity of the test compounds to inhibit colchicine binding to tubulin was measured as described above (tubulin polymerization effect) except that the reaction mixtures contained 1 μM tubulin, 5 μM [3H]colchicine, and 5 μM test compound.
4.7. Flow cytometry
They were trypsinized, washed in PBS and centrifuged at 2000 rpm for 5 min. The pellet was then resuspended in 500 μL of staining solution (containing 5 μL Annexin V-PE and 5 μL PI in Binding Buffer), mixed gently and incubated for 15 min at room temperature (15–25 °C) in dark. The samples were then read in a FACScalibur flow cytometer (USA) at 488 nm excitation. Analyses were performed by the software supplied in the instrument.
4.8. Docking simulations
The three-dimensional X-ray structure of tubulin (PDB code: 1SA0) was chosen as the template for the modeling study of compound 13. The pdb file about the crystal structure of the tubulin domain bound to colchicine (CN2700) (1SA0.pdb) was obtained from the RCSB protein data bank (http://www.pdb.org). The molecular docking procedure was performed by using CDOCKER protocol for receptor-ligand interactions section of Discovery Studio 3.5 (Accelrys Software Inc, Organic & Biomolecular Chemistry Page 20 of 3419 San Diego, CA).40 All bound water and ligands were eliminated from the protein and the polar hydrogen was added. The whole tubulin complex was defined as a receptor and the site sphere was selected based on the ligand binding location of colchicine (CN2700), then the CN2700 molecule was removed and compound 13 was placed during the molecular docking procedure. Types of interactions of the docked protein with ligand were analyzed after the end of molecular docking.4.9. 3D-QSAR
Ligand-based 3D-QSAR approach was performed by QSAR software of DS 3.5 (Discovery Studio 3.5, Accelrys, Co. Ltd). The training sets were composed of inhibitors with the corresponding pIC50 values which were converted from the obtained IC50 (μM), and test sets comprised compounds of data sets as list in Table 3. All the definition of the descriptors can be seen in the “Help” of DS 3.5 software and they were calculated by QSAR protocol of DS 3.5. The alignment conformation of each molecule was the one with lowest interaction energy in the docked results of CDOCKER. The predictive ability of 3D-QSAR modeling can be evaluated based on the cross-validated correlation coefficient, which qualifies the predictive ability of the models. Scrambled test (Y scrambling) was performed to investigate the risk of chance correlations. The inhibitory potencies of compounds were randomly reordered for 30 times and subject to leave-one-out validation test, respectively. The models were also validated by test sets, in which the compounds were not included in the training sets. Usually, one can believe that the modeling is reliable, when the R2 for test sets is larger than 0.6, respectively.Acknowledgements
The work was financed by a grant from Major Projects on Control and Rectification of Water Body Pollution (no. 2011ZX07204-001-004), and supported by “PCSIRT" (IRT1020).Notes and references
- A. Müsch, Traffic, 2004, 5, 1–9 CrossRef PubMed.
- M. A. Jordan and L. Wilson, Nat. Rev. Cancer, 2004, 4, 253 CrossRef CAS PubMed.
- A. Jordan, J. A. Hadfield, N. J. Lawrence and A. T. McGown, Med. Res. Rev., 1998, 18, 259 CrossRef CAS.
- E. Hamel, Med. Res. Rev., 1996, 16, 207 CrossRef CAS.
- R. Dhamodharan, M. A. Jordan, D. Thrower, L. Wilson and P. Wadsworth, Mol. Biol. Cell, 1995, 6, 1215 CrossRef CAS.
- D. Panda, H. P. Miller, K. Islam and L. Wilson, Proc. Natl. Acad. Sci. U. S. A., 1997, 94, 10560 CrossRef CAS.
- J. M. Gerdes and N. Katsanis, Hum. Mol. Genet., 2005, 14, R291 CrossRef CAS PubMed.
- C. Dumontet and M. A. Jordan, Nat. Rev. Drug Discovery, 2010, 9, 790 CrossRef CAS PubMed.
- P. Singh, K. Rathinasamy, R. Mohan and D. Panda, IUBMB Life, 2008, 60, 368 CrossRef CAS PubMed.
- R. B. Ravelli, B. Gigant, P. A. Curmi, I. Jourdain, S. Lachkar, A. Sobel and M. Knossow, Nature, 2004, 428, 198 CrossRef CAS PubMed.
- B. P. Chatterji, B. Jindal, S. Srivastava and D. Panda, Expert Opin. Ther. Pat., 2011, 21, 167 CrossRef CAS PubMed.
- D. M. Drake and D. W. Pack, J Pharm. Sci., 2008, 97, 1399 CrossRef CAS PubMed.
- J. K. Lam, S. P. Armes and S. Stolnik, J. Drug Targeting, 2011, 19, 56 CrossRef CAS PubMed.
- K. N. Bhalla, Oncogene, 2003, 22, 9075 CrossRef CAS PubMed.
- L. G. Wang, X. M. Liu, W. Kreis and D. R. Budman, Cancer Chemother. Pharmacol., 1999, 44, 355 CrossRef CAS PubMed.
- G. R. Pettit, S. B. Singh, E. Hamel, C. M. Lin, D. S. Alberts and D. Garcia-Kendal, Experientia, 1989, 45, 209 CrossRef CAS.
- C. M. Lin, H. H. Ho, G. R. Pettit and E. Hamel, Biochemistry, 1989, 28, 6984 CrossRef CAS.
- J. Chang, H. Hsieh, C. Chang, K. Hsu, Y. Chiang, C. Chen, C. Kuo and J. Liou, J. Med. Chem., 2006, 49, 6656 CrossRef CAS PubMed.
- E. J. Park, H. R. Park, J. S. Lee and J. Kim, Planta Med., 1998, 64, 464 CrossRef CAS PubMed.
- A. C. Claude, C. L. Jean, T. Patric, P. Christelle, H. Gerard, J. C. Albert and L. D. Jean, Anticancer Res., 2001, 21, 3949 Search PubMed.
- S. K. Kumar, H. Erin, P. Catherine, G. Halluru, N. E. Davidson and S. R. Khan, J. Med. Chem., 2003, 46, 2813 CrossRef CAS PubMed.
- K. L. Lahtchev, D. I. Batovska, S. P. Parushev, V. M. Ubiyvovk and A. A. Sibirny, Eur. J. Med. Chem., 2008, 43, 2220 CrossRef CAS PubMed.
- Y. Qian, G. Ma, Y. Yang, K. Cheng, Q. Zheng, W. Mao, L. Shi, J. Zhao and H. Zhu, Bioorg. Med. Chem., 2010, 18, 4310 CrossRef CAS PubMed.
- H. H. Ko, L. T. Tsao, K. L. Yu, C. T. Liu, J. P. Wang and C. N. Lin, Bioorg. Med. Chem., 2003, 11, 105 CrossRef CAS.
- H. Matsuda, T. Morikawa, S. Ando, T. Iwao and Y. Mas-ayuki, Bioorg. Med. Chem., 2003, 11, 1995 CrossRef CAS.
- C. M. Lin, S. B. Singh, P. S. Chu, R. O. Dempcy and J. M. Schmidt, Mol. Pharmacol., 1988, 34, 200 CAS.
- M. A. Jordan and L. Wilson, Nat. Rev. Cancer, 2004, 4, 253 CrossRef CAS PubMed.
- B. A. Teicher, Clin. Cancer Res., 2008, 14, 1610 CrossRef CAS PubMed.
- S. Honore, E. Pasquier and D. Braguer, Cell. Mol. Life Sci., 2005, 62, 3039 CrossRef CAS PubMed.
- J. R. Dimmock, D. W. Elias, M. A. Beazely and N. M. Kandepu, Curr. Med. Chem., 1999, 6, 1125 CAS.
- V. Peyrot, D. Leynadier, M. Sarrazin, C. Briand, M. Menendez, J. Laynez and J. M. Andreu, Biochemistry, 1992, 31, 11125 CrossRef CAS.
- S. Ildiko and M. Marilena, Bioconjugate Chem., 2009, 20, 665 Search PubMed.
- Y. Yulia and D. Scaffidi, Inorg. Chem., 2010, 49, 5678 Search PubMed.
- Y. Luo, K. Qiu, X. Lu, K. Liu, J. Fu and H. Zhu, Bioorg. Med. Chem., 2011, 19, 4730 CrossRef CAS PubMed.
- D. Li, F. Fang, J. Li, Q. Du, J. Sun, H. Gong and H. Zhu, Bioorg. Med. Chem. Lett., 2012, 22, 5870 CrossRef CAS PubMed.
- J. Dalmolen, T. D. Tiemersma Wegman, J. W. Nieuwenhuijzen, M. van der Sluis, E. van Echten, T. R. Vries, B. Kaptein, Q. B. Broxterman and R. M. Kellogg, Chemistry, 2005, 11, 5619 CrossRef PubMed.
- C. Selvaraj, S. K. Tripathi, K. K. Reddy and S. K. Singh, Curr. Trends Biotechnol. Pharm., 2011, 5, 1104 CAS.
- I. Vermes, C. Haanen, H. Steffens-Nakken and C. Reutelingsperger, J. Immunol. Methods, 1995, 51, 184 Search PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2007, 64, 112 CrossRef PubMed.
- G. Wu, D. H. Robertson, C. L. Brooks and M. Vieth, J. Comput. Chem., 2003, 24, 1549 CrossRef CAS PubMed.
Footnotes |
| † CCDC 989497–989499. For crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ra03803g |
| ‡ Both authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2014 |