Interface chemistry engineering in electrode systems for electrochemical energy storage
Lei Yu
a,
Zhongyu Qiana,
Nannan Shia,
Qi Liu*a,
Jun Wang
*ab and
Xiaoyan Jinga
aKey Laboratory of Superlight Material and Surface Technology, Harbin Engineering University, Harbin, 150001, P. R. China. E-mail: zhqw1888@sohu.com; Fax: +86 451 8253 3026; Tel: +86 451 8253 3026
bMinistry of Education and Institute of Advanced Marine Materials, Harbin Engineering University, Harbin, 150001, P. R. China
First published on 13th August 2014
Abstract
Although high power electrochemical capacitors and high energy batteries have some differences in the charge storage mechanism, high-efficiency energy storage requires an excellent interface between the electronic-transporting phase (electrode) and the ionic-transporting phase (electrolyte). Considerable amounts of attention have been paid to the interface and the entire electrode system to solve all kinds of technological challenges for their development. We introduce two powerful strategies for a well-controlled interface (step-by-step nano-architectures grown on substrates and nanoscale building blocks with spatial precision). Then, some recent advances are focused on for the interface design of current collectors and highlight the interfacial bonding between electrochemically active materials and conductive substrates. Interface chemistry engineering in electrode systems for electrochemical energy storage needs to integrate individual materials components to interface design and optimization. Step-by-step growth provides robust contact between working materials and substrates. Nanoscale building blocks as solution-processable precursors are a potentially low cost alternative and are promising for bulk production. Flexible step-by-step assembly by combining interfacial self-assembly with nanoscale building blocks may be a powerful method for interface chemistry in electrochemical energy storage.
1. Introduction
Electrochemical power sources, as a secure and sustainable energy supply, have been intensively investigated for technological development and good standard of living.1 High power electrochemical capacitors (ECs) and high energy lithium ion batteries (LIBs) are at the forefront of these, to meet the higher requirements of future systems.2 Although they have some difference in the charge storage mechanism (ECs store charges at the surface or in a thin layer of active materials in reversible processes, while, LIBs store charges by the intercalation of Li+ ions from the surface to the bulk of active materials),3 developing new materials and advancing architecture designs of the electrochemical interfaces assign equal importance to them for future energy storage systems.4,5The high-efficiency energy storage requires an excellent interface between the electronic-transporting phase (electrode) and the ionic-transporting phase (electrolyte).6 Nanostructured materials with a shortened ion diffusion pathway benefit high energy devices, high power performance relies on enhanced electron transport related with electronic conductivity and mobility.4 The hybrid or composite materials integrate several types of functional materials and make them synergistically enhance the properties of each component. 3D-architecture design of materials shortens the transport lengths for ions and increases the contact area between the electrode and the electrolyte.5 Heterostructured nanoarchitectures have achieved notable success in scrupulous design of smart hybridization and controllable microstructure for electrochemical energy storage. Superior to traditional core–shell nanostructure or 3D-architecture design, interface chemistry engineering leads to long cycle life, low cost, and a relatively larger energy density. In addition, hybrid interface can modification of each other with reinforcements and fewer inherent limitations.7–9 A range of nanoscale building blocks, such as electric double-layer capacitive (EDLC) graphene or carbon nanotube materials (high-power but relatively low-energy, reasonably high conductivity and relatively high surface-area),10–12 moderate electrochemical conducting polymers,13–15 and pseudocapacitive transition-metal-based oxides or hydroxides (TMOs) or battery materials (high-energy, volume change during electrochemical process and poor electric conductivity),16–18 have gained prominence with high specific surface area and small dimensions. Besides, interface between current collectors and active materials has also attracted increasing interests.19,20
Most traditional research focused on active materials and synthesis, as it has been widely accepted that interface of electrode materials plays a key role during electrochemical process, considerable amounts of attentions have been paid on interface and entire electrode system to solve all kinds of technological challenges for the development.21 Practical applications require balance between capacity and specific capacity. Electrodes should have a large mass loading towards green grid,22–24 and interface should be designed with an optimal physical space.7 Nanocomposites could be achieved with well-controlled interface by two powerful strategies: step-by-step nano-architectures grown on substrates3,25,26(desirable to integrate these hybrid nanocomposites and substrates with robust interface, but is not sufficient in nanoscale spatial precision with limited synthetic methods) and nanoscale building blocks with spatial precision27–29(the fabrication is more flexible, but often lacks suitable interfacial bonding with the substrates by using slurry-pasting). We first review the two promoting ways of fabricating hybrid electrode materials. After that, some recent advances are focused on interface design of current collectors. Next, we highlight the interfacial bonding between electrochemically active materials and conductive substrates. The concluding section is focused on several promising research trends in the electrode architecture design with an outlook.
2. Step-by-step nano-architectures grown on substrates
Step-by-step growing avoids additives and provides robust contact between working materials and substrates, the interval space formed between neighboring makes easy diffusion of ionic-transporting region.26 Some efforts have also been directed at the search from metal oxide materials-conductive matrix to hybrid mixed metal oxides,3,25,26 primary structure grown on substrates should be electrochemically stable and good electrical conductivity, compared with the subsequent structure. Hybrid mixed metal oxides exhibit better in high energy for fast ion transport, but worse electronic-transporting than carbon-based structures.27–39 Step-by-step growing is concordant with interface design of current collectors, poor electronic-transporting phase may rely on the interface of current collectors more,27–39 interface design of current collectors will be discussed later.Heterostructured core–shell nanowire arrays may be one of the most scientific and technological interests. Fabrication of core nanowire arrays with well-defined morphologies is critical, which is often achieved by electrodeposition,32–34 vapor deposition35–37 and hydrothermal synthesis.38,39 Currently, tremendous efforts have been devoted to developing cost-effective and simple methods. For example, Xia et al. presented a simple two-step solution-based method to synthesize transition metal oxide core–shell nanoarrays by combining hydrothermal synthesis and chemical bath deposition, core nanowires could act as backbones to guide the shell materials preferentially deposited, and the core–shell nanoarrays lead to enhanced electrochemical properties (452 F g−1 and 1.35 F cm−2 at 2 A g−1)31 (Fig. 1).
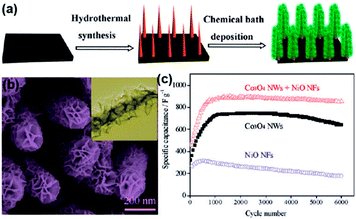 | ||
| Fig. 1 (a) Schematic illustration of the two-step synthesis of metal oxide core–shell nanowire arrays directly on various substrates. (b) Scanning electron micrograph and transmission electron micrograph (inset) of metal oxide core–shell nanowire arrays. (c) Cycling performances of Co3O4/NiO nanowire arrays. (Reproduced from ACS Nano, 2012, 6, 5531–5538. Copyright 2012, American Chemical Society.31) | ||
Hydrothermal approaches may be facile for one-dimensional (1D) nanoarrays directly on various substrates and be spoke self-assembled structures.40–43 As another point of view, it is a kind of technique for tailoring the interface of the matrix. Nanowire arrays are designed to guarantee a fast ion and electron transfer, so there still exists some nano-architectures may be promising for electrochemical energy storage, such as nanoflake arrays and networked nanocoatings.44–47 More high-effective interfaces should be explored with three-dimensional porous structures and robust interface from self-assembled nanomaterials.48–52 Nanoflake arrays seem to exhibit poor microstructures compared with nanowire arrays, but the fabrication is flexible and easy to scale up. Zhang et al. presented a simple template-free solution-based method combined with a post annealing treatment, it is successfully developed to grow interconnected mesoporous NiCo2O4 nanosheets on various conductive substrates, the integrated electrodes exhibit an ultrahigh specific capacitance (1743.4 F g−1 and 2.09 F cm−2 at 8.5 mA cm−2) and excellent cycling stability at a high charge/discharge current density (17.1% loss, 3000 cycles,at 25 mA cm−2).53 (Fig. 2) NiCo2O4 has better electrical conductivity and higher electrochemically activity compared with NiO and Co3O4, it could be good backbones to guide the shell materials grown.
 | ||
| Fig. 2 Scanning electron micrographs of the NiCo2O4 nanosheets on Ni foam after cycling for 3000 cycles with a current density of 25 mA cm−2. (Reproduced from Adv. Mater., 2013, 25, 976–979. Copyright 2013, Wiley-VCH.53) | ||
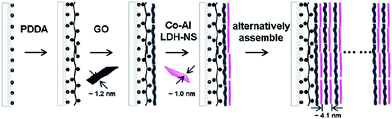 | ||
| Fig. 3 Schematic of layer-by-layer assembly process for multilayer films of positively charged Co–Al LDH nanosheets and negatively charged graphene oxide nanosheets. (Reproduced from Langmuir, 2012, 28, 293–298. Copyright 2012, American Chemical Society.55) | ||
Step-by-step growing promotes a binder- and conductive agent-free robust adhesion. However, these electrodes often have a small mass loading compared with slurry-pasting,42,43,53 the technique needs to be considered with interface design of current collectors to integrate the interface of electrodes system. Whether growing or not is not the foremost, robust adhesion with substrates should be considered first. It can be achieved by electrostatic sequential adsorption, such as layer-by-layer (LBL) assembly, Langmuir–Blodgett and electrophoretic deposition.54 Dong et al. reported the fabrication of flexible films of Co–Al layered double hydroxide (LDH) nanosheets/graphene oxide via LBL assembly process (1204 F g−1 and 90 F cm−2 at 5 mV s−1, 40 bilayers; over 99% after 2000 cycles, at 20 A g−1) (Fig. 3).55 LBL assembly is a powerful tool to build well organized layered films with finely controlled film thickness and uniformity, but it may be difficult for a large mass loading. However, it is desirable for step-by-step to grow developing towards more flexible step-by-step assembly. It can be inferred from the related discussion that the first step is difficult and limited, but the second step is various and flexible.
3. Nanoscale building blocks with spatial precision
3.1 Planar or graphene-based nanostructures
Flexible solution-based methods have scrupulous design of nanoarchitectures and smart hybridization. Graphene oxide (GO), as a promising solution-processable precursor for the bulk production of reduced graphene oxide (rGO) and potentially low cost alternative, is derivative by functional groups for growth of inorganic nanoparticles to afford strongly coupled hybrids.56–58 High energy active materials, such as TMOs, exhibit electrochemically active with electrolyte, but poor electronic-transporting. During an electrochemical process, phase change is estimated from surface to bulk entirely towards high energy. For better electronic-transporting, electrochemically stable phase is needed. Strongly coupled TMOs–graphene hybrid materials achieve these with graphene acted as stable electric conductive phase and TMOs acted as active materials for high energy. The self-assembly of nanoparticles anchored on GO nanosheets could be directed with surfactants-assisted,59–61 (Fig. 4) solvent-assisted dispersion of graphene-based material or hydrothermal,62–64 which opens a promising avenue for replacing the framework carbon atoms of graphene.65 Wang et al. coerced multiple phases of SnO2 and graphene into deterministic nanostructured materials by self-assembly (760 mA h g−1, at 80 mA g−1).59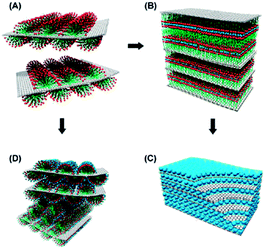 | ||
| Fig. 4 Schematic illustrations of the ternary self-assembly approach to ordered metal oxide/graphene nanocomposites. (A) Adsorption of surfactant hemimicelles on the surfaces of the graphene causes its dispersion in surfactant micelles in an aqueous solution. (B) The self-assembly of anionic sulfonate surfactant on the graphene surface with oppositely charged metal cation species and the transition into the lamella mesophase nanocomposites. (C) Metal oxide/graphene layered nanocomposites composed of alternating layers of metal oxide nanocrystals and graphene stacks after crystallization of metal oxide and removal of the surfactant. (D) Self-assembled hexagonal nanostructure of metal oxide precursor with nonionic surfactants on graphene stacks. (Reproduced from ACS Nano, 2010, 4, 1587–1595. Copyright 2010, American Chemical Society.59) | ||
Stable, single and few-atom-thick two-dimensional (2D) nanomaterials beyond graphene have been one of the most extensively studied to exhibit fascinating and technologically useful properties, such as charge transport confined to a plane and high electron mobility.66 Liquid exfoliation of layered compounds are now considered to be excellent candidates for future electronic applications.67,68 For example, positively charged layered double hydroxide nanosheets and some negatively charged oxide nanosheets could be obtained by using formamide and quaternary amine ion, respectively.69–72 Time-consuming and possible surface residues of foreign ions are quite unfavorable, Feng et al. took advantage of an intermediate intercalated compound precursor of VS2·NH3 (4.76 F cm−2 and 317 F cm−3 at 0.2 A m−2, area: 11.52 cm2, thickness: 150 nm).73 Besides, “Bottom Up” strategies process a efficient route for liquid exfoliation.74–76 Flocculation occurs when electrolytes (counterions) added to colloidal nanosheets.67 Directed assembly of positively and negatively charged nanosheets can be achieved to construct sandwich lamellar systems, it is possible to tailor superlattice-like assemblies by incorporating 2D nanocrystal building blocks.67,77
3.2 Interpenetrating networks of 1D nanomaterials
1D carbon nanotubes (CNTs) can be viewed as graphene sheets rolled up into nanoscale tubes.78 CNTs have gained prominence as nanoscale building blocks for dispersing the active materials to prevent them from agglomerating, interpenetrating networks of 1D CNT-based nanomaterials also afford an efficient electronic pathway to provide better electrical contacts.4,79 Hierarchical interconnected pore channels can be also achieved by hybrid CNTs and V2O5 nanowires (792 F g−1, 3.8 mg cm−2 for lithium ion ECs; ∼400 F g−1, 1–3 mg cm−2 for sodium ion ECs).80,81 (Fig. 5) High-power CNTs and high energy V2O5 are intertwined with each other, the network ensures fast and efficient electronic-transporting.11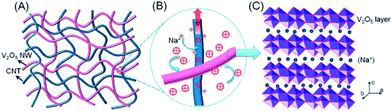 | ||
| Fig. 5 Schematic of (A) a nanocomposite consisting of interpenetrating networks of V2O5 nanowires and CNTs, (B) intimate contacts between the V2O5 nanowire and CNTs facilitating charge transport, and (C) Na+ intercalation within the V2O5 layer structure. (Reproduced from ACS Nano, 2012, 6, 4319–4327. Copyright 2012, American Chemical Society.81) | ||
One dimension nanomaterials are easy to build networks with short ion diffusion paths and better utilization.11 Among various methods, template-directed and hydrothermal synthesis has been a powerful route with simplicity and great variability in controlling the chemical composition and morphology in both lateral and longitudinal dimensions.82–84 Macroscopic-scale assembled nanowires thin films promote the interface-induced and external fields assisted technology, for example, Langmuir–Blodgett Technique, evaporation-induced, microfluidic flow, bubble-blowing, electrospinning, magnetic field-assisted.85
3.3 Three-dimensional gel frameworks
Three-dimensional (3D) graphene gel frameworks can be cheaply produced on a large scale from graphene oxide by hydrothermal or additives,86–89 The 3D structures provide high specific surface areas, strong mechanical strengths and fast mass and electron transport kinetics.90 Hierarchical macro- and mesoporous graphene aerogels (GAs) can be also fabricated, the macropores can act as a bulk buffering reservoir for electrolytes diffusion, while the mesopores can provide a larger accessible active surface area, and micropores can continuously increase charge accommodation.91 (Fig. 6) Hierarchical porous structures also benefit TMOs, large pores increase ion transport, small pores increase volumetric energy densities.92 Hydrogel interpenetrates solid-state with liquid-state, that is to say, it interpenetrates efficient ion and electron transport. | ||
| Fig. 6 Fabrication of hierarchical macro- and mesoporous GAs–SiO2 frameworks: (i) electrostatic adsorption and assembly of ofcetyltrimethyl ammonium bromide (CTAB) on the surface of 3D GAs, (ii) tetraethoxysilane hydrolysis for nucleation and growth of mesoporous silica on the surface of CTA+-adsorped GAs, and (iii) CTAB removal through ethanol washing, drying, and thermal annealing. (Reproduced from J. Am. Chem. Soc., 2012, 134, 19532–19535. Copyright 2012, American Chemical Society.91) | ||
 | ||
| Fig. 7 Scanning electron micrographs (A, C and E) and transmission electron micrographs (B, D and F) images of the three samples: (A and B) single-shelled Co3O4 hollow spheres, (C and D) double-shelled spheres, and E,F) triple-shelled spheres. (Reproduced from Adv. Funct. Mater., 2010, 20, 1680–1686. Copyright 2010, Wiley-VCH.109) | ||
In addition, Worsley et al. utilized carbon to knit together graphene sheets into a macroscopic 3D structure with superior electrical conductivity. Graphene aerogel synthesis was carried out by sol–gel polymerization of resorcinol and formaldehyde, followed by pyrolysis.93 3D Graphene-based compounds can be prepared in situ reduced with self-assembly and embedding of components.94–96 Hydrothermal carbonization and pyrolysis are rising techniques for the synthesis of porous carbonaceous materials.97–99 Hence, an innovative approach is to incorporate metal oxide nanoparticles to the porous carbon materials.8,100,101
3.4 Nanomaterials-assembled hierarchical microstructures
Various methodologies have been developed to achieve self-assemblied special nanostructure to make easy diffusion of ionic-transporting region.102–107 Recently, multishelled hollow structures have received more attention from potential applications.102,108 Multishell hollow Co3O4 spheres composed of oriented self-assembled nanosheets were synthesized via a hydrothermal method, double-shelled spheres benefits superior electrical conductivity and high structural stability with superior void-space-utilizing rate during volume change during Li+ insertion–extraction (Fig. 7).109 Besides, Wang et al. reported a facile “pumpkin-carving” strategy for the production of single-crystal CoSn(OH)6 nanoboxes with a uniform size.102Hydrotalcite-like compounds consist of positively charged brucite-like host layers and charge balancing anions in the interlayer, α-hydroxides are reported to be isostructural with hydrotalcite-like compounds, which have a larger interlayer than that of the brucite-like structure.110,111 The intercalated anions are exchangeable, thus making it possible to adjust the interlayer spacing.110 Wang et al. reported a novel strategy of fabricating conical structures be formed by the rolling-up of layered α-cobalt hydroxide materials, the lower size limit of accessible hollow spaces in cobalt hydroxide-based electrochemical capacitors through an anion exchange reaction studying.112,113 Nanomaterials-assembled hierarchical microstructures construct an excellent interface for ionic-transport, but fail to provide robust adhesions with matrixes, which lead to a poor electron-transporting phase.
3.5 Surface coating
Coating the surface of the electrode material is a common technique used for improving the functionality and performance of the electrode material. For the lithium ion batteries, carbon coating is one of the most popular means of easily enhancing the electronic conductivity of electrode materials, and can also stabilize SEI films.114–116 Moreover, surface coatings onto cathode material with oxides (NiO or MnO2) can essentially shield from direct exposure to the electrolyte solution, improving the structural integrity of the cathode material and suppressing phase transitions.117,118 For the supercapacitors, a conducting polymer such as polypyrrole (PPy) usually is used to coat the surface of the electrode materials to, which lead to significantly improved electron transport within every nanostructures. PPy was chosen not only because it has greater density and better degree of flexibility, but also itself can undergo a fast redox reaction to store charge.1194. Interface design of current collectors
4.1 Microstructured interface design
3D porous current collectors have excellent interfaces between the electronic-transporting phase and the ionic-transporting phase, while, flat current collectors are difficult for electrolyte penetration.120 (Fig. 8) 3D porous conductive materials, such as carbon paper and carbon cloth, are promising for high-performance flexible electrodes.121–124 Similar to EC materials, for current collectors with high electronic conductivity and mobility, only surface or a thin layer around the electrode–electrolyte interface play a key role, microstructured interface design of current collectors is vital for meet the requirements of short transport lengths and large energy capacities with an optimal physical space.7,125 When slurry-pasting is discussed, low specific capacity caused by the extra weight of additives and dead volume is obvious drawbacks.26 these can be also caused by a poor interfacial design of current collectors. Zhang et al. reported a self-assembled bicontinuous bulk electrode concept consisting of an electrolytically active material sandwiched between the electrolyte and the current collectors with polystyrene spheres template-assisted electrodeposition.126 Gowda et al. reported 3D nanoporous Au nanowire current collectors for thin film micro batteries, vertically aligned gold–silver nanotube arrays were fabricated using a template-assisted electrodeposition technique followed by treated with concentrated nitric acid.127 Chemical-ecthing with nitric acid is a powerful way for nanoporous electrodes.128,129 | ||
| Fig. 8 Design and fabrication of 3D porous current collectors filled with the battery electrode material. (a) (left): Highly conductive fibrous network after coating polyester fibers with carbon nanotubes and (right): Porous conductor filled with battery materials, where the conductive polyester fiber network functions as an effective current collector and the organic electrolyte penetrates throughout the entire structure effectively. (b) By comparison, the battery electrode material is coated on the surface of the flat current collector in the traditional architecture. (Reproduced from Adv. Energy Mater., 2011, 1, 1012–1017. Copyright 2011, Wiley-VCH.120) | ||
Ideal electrode architectures provide three-dimensional interpenetrating efficient ion and electron transport.126 Attempts at novel electrode design have been extensively made from conductive matrixes to integrate materials hybrid nanostructures.30 Step-by-step growing is concordant with the microstructured interface design of current collectors to assign to bridge the performance for electrode design. Step-by-step growing of battery materials is suitable for high-energy devices, while, microstructured interface design of current collectors may benefit high-power devices.130
4.2 Surface modification and functionalization
Textile fibers have a hierarchical structure with complicated surface morphology, each fiber is comprised of multiple individual cotton fibrils, which are in turn composed of multiple microfibrils bundled, functional groups cause the fibers to swell in polar solutions. Flexible and porous textiles are used as supporters to make nanosystems electrodes,132 and provide an effective three-dimensional (3D) framework for a large mass loading of electrochemical active materials. Porous conductors could be made with carbon nanotube or graphene coated,131–134 the fabrication process is simple and scalable, similar to those widely used for dyeing fibers and fabrics in the textile industry. (Fig. 9) Cotton T-shirt textiles could be converted into activated carbon textiles for energy storage applications.135 Inspired of these, hierarchical macroporous sponges and flexible paper have been designed to conductive substrates.136–139 Polypyrrole-coat papers could be fabricated by a simple “soak and polymerization”.140 Hu et al. demonstrated highly conductive paper for energy-storage devices with single-walled CNTs and Ag nanowires integrated by solution-based processes.141 | ||
| Fig. 9 Porous textile conductor fabrication. (a) Schematic of SWNTs wrapping around cellulose fibers to form a 3D porous structure. (b) Conductive textiles are fabricated by dipping textile into an aqueous CNT ink followed by drying in oven at 120 °C for 10 min. (c) A thin, 10 cm × 10 cm textile conductor based on a fabric sheet with 100% cotton and Rs of 4 Ω sq−1. (d) SEM image of coated cotton reveals the macroporous structure of the cotton sheet coated with CNTs on the cotton fiber surface. (e) SEM image of fabric sheet coated with CNTs on the fabric fiber surface. (f) High-magnification SEM image shows the conformal coating of CNT covering and bridging between the fabric fibers. (g) TEM image of CNTs on cotton fibers. (Reproduced from Nano Lett., 2010, 10, 708–714. Copyright 2010, American Chemical Society.131) | ||
Alignments of nanocrystalline phases with different morphologies and microstructures show substantial differences in electrochemical performances, substrate dependent self-organization of mesoporous cobalt oxide nanowires with remarkable pseudocapacitance were reported.142 Interfacial designs for electron and ion transfer are affected by chemical and physical properties of substrates, it implies the importance surface modification and functionalization.143–145 A high mass loading of active materials usually leads to an increased electrode resistance. To solve these critical problems, Yu et al. developed a “3D conductive wrapping” method.146
4.3 Self-supporting materials
Papers are excellent candidates for substrates of flexible energy-storage applications and miniature devices, carbon nanotube/cellulose papers could be used in all-solid-state flexible supercapacitors.147–149 Flexible CNT or graphene thin films could be prepared by filtration,149–151 incorporating these with transition metal oxides or conductive polymers is a promising approach to further achieve better electrochemical performances of devices.152,153 (Fig. 10) Recently, macroscopic GO membranes were prepared by evaporating GO at a liquid–air interface.154 Electrochemically active materials serve as current collectors in self-supporting electrodes, no additional conductive matrix exists. Portable electronic devices develop in the trend of being small, thin, lightweight, flexible and even roll-up, flexible and lightweight devices need to provide much higher energy and power with less device mass, these could be achieved by combining thin films electrodes and polymer gel electrolytes.141–148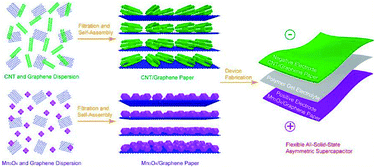 | ||
| Fig. 10 Illustration of the fabrication process for flexible all-solid-state asymmetric supercapacitors based on polymer gel electrolyte and free-standing paper electrodes. (Reproduced from ACS Appl. Mater. Interfaces, 2012, 4, 7020–7026. Copyright 2012, American Chemical Society.153) | ||
Ultralight and highly porous CNT or graphene aerogels may be another exciting candidates for electrically conducting aerogels,155,156 the fabrication could be incorporated with transition metal oxides or conductive polymers for enhanced electrochemical performances.157–159 Folded structured graphene paper from 3D graphene aerogels is also adaptable for high performance electrode materials.160 Inspired by wearable electronic devices with textiles, easily woven fibrous electrodes could be prepared with CNT yarn infiltrated with polyaniline nanowire arrays.161 Wet-spinning assemblies of macroscopic graphene fibers may be fabricated the same as fiber springing in the future.162–164 Flexible and conductive fibers make wearable electrochemical storage devices feasible by utilizing nanotechnology, if integrated with stretchable energy generation to a self-powering nanosystem, wearable electrochemical storage devices could work when we walk and move.165–168
5. Interfacial bonding between working materials and substrates
5.1 Binders and additives
Slurry-pasting of active materials, conductive additives and polymeric binders onto the current collector is a traditional treatment for electrodes, however, random distribution of the constitutive phases has obvious drawbacks including poor electron transport and extra weight of additives.26 Active materials ensure high energy, conductive additives guarantee high electron transport and power, binders keep structural stability during the electrochemical process. Selecting binders should be considered with interfacial bonding and high performance. For example, sodium alginate may be a good binder compared with polyvinylidene fluoride for lithium ion battery.169 The excellent electrode performance caused by the sodium alginate is not only owing to without react between sodium alginate and electrolyte, but also electrode porosity. Sarkar et al. synthesized NH4V4O10 along with CMC/alginate binder.170 It delivers discharge capacity of 200 mA h g−1 at very high current rate of 1000 mA g−1 and completely retains its original discharge state at low current rate of 100 mA g−1 rate, whereas PVDF-based cathode delivers discharge capacity of 125 mA h g−1 at same current rate. Liu et al. prepared an alginate hydrogel binder which leads to a remarkable improvement in the electrochemical performance of the Si/C anode of a Li-ion battery.171 Besides, conductive binders combine conductive additives with polymeric binders to tailor the conduction band and improve the mechanical binding force.172,173 Wang et al. built a cross-link reaction between active materials coated with polydopamine and polyacrylic acid to form a robust network of covalent bonds in electrodes20 (Fig. 11).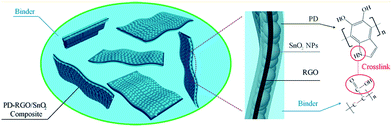 | ||
| Fig. 11 Illustration of the system-level strategy of the fabrication of rGO–SnO2 composite-based anode for lithium ion battery. (Reproduced from Nano Lett., 2013, 13, 1711–1716. Copyright 2013, American Chemical Society.20) | ||
Sometimes, additives may improve the performance. For instance, sodium alginate could direct nanofiber networks of polyaniline,174 coating constituents phase-separate from battery materials could lead to a high-power performance,175 polyvinyl alcohol gel could stabilize vanadium oxide nanowire for pseudocapacitors.176 Heat treatments in air for binders burn-out and active materials sintered may be a powerful binder-free technique for porous metal oxides.177,178 It promotes TMOs nanoscale building blocks to construct hierarchically porous bulks.
5.2 Weak interactions
Nanoelectrodes have higher electrode–electrolyte contact areas and short path lengths for electronic and ionic transport.92 Various flexible techniques are developed for nanomaterials, however, most of these rely on slurry-pasting. Slurry-pasting is more suitable for nanomaterials than nanoelectrodes, inferior packing of particles leading to lower volumetric energy densities.92 Robust contacts between working materials and substrates are pivotal. Step-by-step growing promotes a additive-free robust adhesion with current collectors with a small mass loading.Co–Al LDH nanosheets–GO composite could be produced by simply mixing dispersions of each component to maximize the contact area of graphene and nanosheets and to optimize the utilization rate of the nanosheets as active materials (1031 F g−1 1 mg cm−2, at 1 A g−1; nearly 100% after 6000 cycles, at 20 A g−1), (Fig. 12) these conductive additive-free assembled materials still needs binders to contact with current collectors.179,180 It could be achieved by electrophoretic deposition or self-assembly thin film.137,181 It implies interfacial assembly with current collectors is operable. Robust adhesion with substrates and smart hybrid can be achieved by layer-by-layer assembly, however, to have a large mass loading is time-consuming, co-assembly of mixed nano-objects with different sizes and/or shapes represents a fundamentally interesting topic, co-assembly of nanowires and GO nanosheets directs unidirectional self-alignment of nanowires at the air–water interface,182 co-assemblies of multicomponent colloids remain to be attractive for applications in electronics and electrodes.183
 | ||
| Fig. 12 (a) Schematic of the formation and structure of Co–Al LDH nanosheets/graphene oxide composite. (b) Digital photographs of (left) an aqueous dispersion of LDH nanosheets, (middle) an aqueous dispersion of graphene oxide, and (right) a mixture of LDH nanosheets and graphene oxide.179 | ||
Textiles could absorb polar solvents, the interactions of functional groups with solutes, the weak interactions combine textiles with solutes when the solvents are evaporated.145 Various interfacial assemblies of nanomaterials have been improved, it has been widely accepted interfacial assembly is solvent-assisted and substrate-dependent.184–186 Integrated system could be built up with dispersion of nanomaterials in suitable solvents and substrate surface modification. Filtration contains structure-induced interfacial assembly; transition metal oxide nanowire/CNT intertwined mesh could be prepared by a filtration method with the nanowires trapped on the CNT films.187
As another point of view, instead of building robust interfaces with substrates, dispersing active materials in a liquid electrolyte may meet the demand for batteries of higher energy and power. Flow architecture is named as semi-solid flow battery, Duduta et al. designed diffusion-limited cluster aggregation of conductive nanoparticles as percolating conductor networks and “hit and stick” behavior that forms fractal particle stabilizing the larger particles from settling out of suspension, the semi-solid flow battery shows an increasing energy density.188–190 It can be inferred that the stability of suspensions and conductor networks plays a pivotal role, during the electrochemical process actives materials adhering to electrodes are undesirable. Molecules and ions in solutions are stable and homogenous dispersed, active materials homogenous dispersed will be better for electrochemical devices. Yang et al. proposed a new room-temperature lithium/polysulde semi-liquid battery, no insoluble phases exist in the proof-of-concept battery with high energy density.191
5.3 Interconnected structures
Nanostructured materials with well-defined morphologies have obtained success, while, nanostructured electrodes with efficient electron and ion pathways are difficult to control. Nanostructured electrodes could be prepared by attaching active materials on the 3D networked scaffold with robust interfacial bonding between working materials and substrates. However, ideal 3D nanoarchitectured electrode should be interpenetrating active materials and conductive materials for electron and ion networks.85,82 Chen et al. applied an H2 gas bubble dynamic template route to design 3D nanoarchitectured metal electrodes, followed by a heat treatment for interpenetrated metal/oxide (721.7 mA h g−1, at 1 C rate).19 (Fig. 13) Wang et al. proposed a strategy construct a free-standing, hierarchically porous carbon with GO sol–gel dropped into the nickel-foam and carbonized for Li–O2 batteries.192 Various 3D interpenetrated nanoarchitectures may be constructed with chemical bath deposition or other solution-based methods.193–198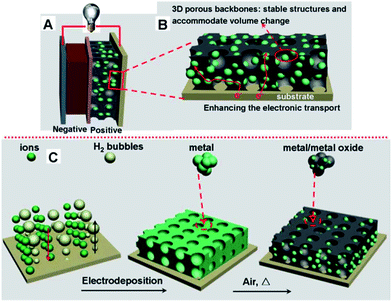 | ||
| Fig. 13 (A) A schematic of lithium ion batteries, including the porous micro/nanostructured interconnected metal/metal oxide electrode. (B) A structural illustration of a metal/metal oxide electrode. (C) The metal/metal oxide electrode was fabricated via an H2 gas bubble dynamic template route.19 | ||
6. Future outlook
Slurry-pasting contains the preparation of the slurry and coating with current collectors, and could be seen as a rough technique to a certain extent. If active materials could be dispersed in the slurry homogenously and steadily, the slurry could be used for semi-solid flow batteries. If the components in the slurry could be controlled with a scrupulous design, interfacial assembly could be achieved with a robust adhere with the matrix. Step-by-step growing provides a robust structure with restricted conditions, while, flexible nanoscale materials need to be integrated with interfacial assembly. Slurry should be studied for flexible step-by-step assembly by combining interfacial self-assembly with nanoscale build blocks.Acknowledgements
This work was supported by National Natural Science Foundation of China (21353003), Special Innovation Talents of Harbin Science and Technology (2013RFQXJ145), Fundamental Research Funds of the Central University (HEUCFZ), Natural Science Foundation of Heilongjiang Province (B201316), Program of International S&T Cooperation special project (2013DFA50480), and the fund for Transformation of Scientific and Technological Achievements of Harbin (2013DB4BG011).Notes and references
- J. B. Goodenough, Acc. Chem. Res., 2012, 46, 1053–1061 CrossRef PubMed.
- P. Simon and Y. Gogotsi, Nat. Mater., 2008, 7, 845–854 CrossRef CAS PubMed.
- J. Jiang, Y. Li, J. Liu, X. Huang, C. Yuan and X. W. Lou, Adv. Mater., 2012, 24, 5166–5180 CrossRef CAS PubMed.
- S. Xin, Y. G. Guo and L. J. Wan, Acc. Chem. Res., 2012, 45, 1759–1769 CrossRef CAS PubMed.
- K. Naoi, W. Naoi, S. Aoyagi, J. i. Miyamoto and T. Kamino, Acc. Chem. Res., 2013, 46, 1075–1083 CrossRef CAS PubMed.
- L. Pan, G. Yu, D. Zhai, H. R. Lee, W. Zhao, N. Liu, H. Wang, B. C. K. Tee, Y. Shi, Y. Cui and Z. Bao, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 9287–9292 CrossRef CAS PubMed.
- D. R. Rolison, R. W. Long, J. C. Lytle, A. E. Fischer, C. P. Rhodes, T. M. McEvoy, M. E. Bourga and A. M. Lubers, Chem. Soc. Rev., 2009, 38, 226–252 RSC.
- H. Jiang, J. Ma and C. Li, Adv. Mater., 2012, 24, 4197–4202 CrossRef CAS PubMed.
- Q. Lu, J. G. Chen and J. Q. Xiao, Angew. Chem., Int. Ed., 2013, 52, 1882–1889 CrossRef CAS PubMed.
- M. Pumera, Energy Environ. Sci., 2011, 4, 668–674 CAS.
- H. Jiang, P. S. Lee and C. Li, Energy Environ. Sci., 2013, 6, 41–53 CAS.
- S. Yang, R. E. Bachman, X. Feng and K. Müllen, Acc. Chem. Res., 2012, 46, 116–128 CrossRef PubMed.
- M. Zhou, J. Qian, X. Ai and H. Yang, Adv. Mater., 2011, 23, 4913–4917 CrossRef CAS PubMed.
- B. Babakhani and D. G. Ivey, Electrochim. Acta, 2010, 55, 4014–4024 CrossRef CAS PubMed.
- H. J. Lin, L. Li, J. Ren, Z. B. Cai, L. B. Qiu, Z. B. Yang and H. S. Peng, Sci. Rep., 2013, 3, 1353 Search PubMed.
- Q. F. Zhang, E. Uchaker, S. L. Candelaria and G. Z. Cao, Chem. Soc. Rev., 2013, 42, 3127–3171 RSC.
- W. Wei, X. Cui, W. Chen and D. G. Ivey, Chem. Soc. Rev., 2011, 40, 1697–1721 RSC.
- W. Deng, X. Ji, Q. Chen and C. E. Banks, RSC Adv., 2011, 1, 1171–1178 RSC.
- X. Chen, K. Sun, E. Zhang and N. Zhang, RSC Adv., 2013, 3, 432–437 RSC.
- L. Wang, D. Wang, Z. Dong, F. Zhang and J. Jin, Nano Lett., 2013, 13, 1711–1716 CAS.
- P. Yang and J. M. Tarascon, Nat. Mater., 2012, 11, 560–563 CrossRef CAS PubMed.
- Z. Yang, J. Zhang, M. C. W. Kintner-Meyer, X. Lu, D. Choi, J. P. Lemmon and J. Liu, Chem. Rev., 2011, 111, 3577–3613 CrossRef CAS PubMed.
- F. Y. Cheng, J. Liang, Z. L. Tao and J. Chen, Adv. Mater., 2011, 23, 1695–1715 CrossRef CAS PubMed.
- M. Pasta, C. D. Wessells, R. A. Huggins and Y. Cui, Nat. Commun., 2012, 3, 1149–1155 CrossRef PubMed.
- C. Cheng and H. J. Fan, Nano Today, 2012, 7, 327–343 CrossRef CAS PubMed.
- J. Jiang, Y. Li, J. Liu and X. Huang, Nanoscale, 2011, 3, 45–58 RSC.
- Y. Li, Z. Y. Fu and B. L. Su, Adv. Funct. Mater., 2012, 22, 4634–4667 CrossRef CAS PubMed.
- J. Liu and X. W. Liu, Adv. Mater., 2012, 24, 4097–4111 CrossRef CAS PubMed.
- J. Lee, J. Kim and T. Hyeon, Adv. Mater., 2006, 18, 2073–2094 CrossRef CAS PubMed.
- J. Liu, J. Jiang, C. Cheng, H. Li, J. Zhang, H. Gong and H. J. Fan, Adv. Mater., 2011, 23, 2076–2081 CrossRef CAS PubMed.
- X. Xia, J. Tu, Y. Zhang, X. Wang, C. Gu, X. B. Zhao and H. J. Fan, ACS Nano, 2012, 6, 5531–5538 CrossRef CAS PubMed.
- G.-R. Li, Z.-L. Wang, F.-L. Zheng, Y.-N. Ou and Y.-X. Tong, J. Mater. Chem., 2011, 21, 4217–4221 RSC.
- J. Liu, J. Jiang, M. Bosman and H. J. Fan, J. Mater. Chem., 2012, 22, 2419–2426 RSC.
- R. Liu and S. B. Lee, J. Am. Chem. Soc., 2008, 130, 2942–2943 CrossRef CAS PubMed.
- L. Bao, J. Zang and X. Li, Nano Lett., 2011, 11, 1215–1220 CrossRef CAS PubMed.
- X. Xiao, T. Ding, L. Yuan, Y. Shen, Q. Zhong, X. Zhang, Y. Cao, B. Hu, T. Zhai, Li. Gong, J. Chen, Y. Tong, J. Zhou and Z. Wang, Adv. Energy Mater., 2012, 2, 1328–1332 CrossRef CAS PubMed.
- Z. Tang, C. Tang and H. Gong, Adv. Funct. Mater., 2012, 22, 1272–1278 CrossRef CAS PubMed.
- L. Huang and M. Liu, Nano Lett., 2013, 13, 3135–3139 CrossRef CAS PubMed.
- J. Xu, Q. Wang, X. Wang, Q. Xiang, B. Liang, D. Chen and G. Shen, ACS Nano, 2013, 7, 5453–5462 CrossRef CAS PubMed.
- C. Yuan, L. Yang, L. Hou, J. Li, Y. Sun, X. Zhang, L. Shen, X. Lu, S. Xiong and X. W. Lou, Adv. Funct. Mater., 2012, 22, 2560–2566 CrossRef CAS PubMed.
- C. Zhou, Y. Zhang, Y. Li and J. Liu, Nano Lett., 2013, 13, 2078–2085 CrossRef CAS PubMed.
- Y. Wang, Z. Zhong, Y. Chen, C. Ng and J. Lin, Nano Res., 2011, 4, 695–704 CrossRef CAS PubMed.
- X. C. Dong, H. Xu, X.-W. Wang, Y. X. Huang, M. B. Chan-Park, H. Zhang, L. H. Wang, W. Huang and P. Chen, ACS Nano, 2012, 6, 3206–3213 CrossRef CAS PubMed.
- M. A. Abbasi, Z. H. Ibupoto, M. Hussain, Y. Khan, A. Khan, O. Nur and M. Willander, Sensors, 2012, 12, 15424–15437 CrossRef CAS PubMed.
- W. Dong, T. Zhang, J. Epstein, L. Cooney, H. Wang, Y. Li, Y. B. Jiang, A. Cogbill, V. Varadan and Z. R. Tian, Chem. Mater., 2007, 19, 4454–4459 CrossRef CAS.
- J. B. Wu, Y. Lin, X. H. Xia, J. Y. Xu and Q. Y. Shi, Electrochim. Acta, 2011, 56, 7163–7170 CrossRef CAS PubMed.
- Y. Liu, Y. Chu, Y. Zhuo, M. Li, L. Li and L. Dong, Cryst. Growth Des., 2007, 7, 467–470 CAS.
- J. Liu, X. Huang, Y. Li, X. Ji, Z. Li, X. He and F. Sun, J. Phys. Chem. C, 2007, 111, 4990–4997 CAS.
- J. Y. Lao, J. G. Wen and Z. F. Ren, Nano Lett., 2002, 2, 1287–1291 CrossRef CAS.
- J. Liu, Y. Li, X. Huang, G. Li and Z. Li, Adv. Funct. Mater., 2008, 18, 1448–1458 CrossRef CAS PubMed.
- R. C. Wang and C. H. Li, Cryst. Growth Des., 2009, 9, 2229–2234 CAS.
- M. McCune, W. Zhang and Y. Deng, Nano Lett., 2012, 12, 3656–3662 CrossRef CAS PubMed.
- G. Q. Zhang and X. W. Lou, Adv. Mater., 2013, 25, 976–979 CrossRef CAS PubMed.
- R. Ma and T. Sasaki, Adv. Mater., 2010, 22, 5082–5104 CrossRef CAS PubMed.
- X. Y. Dong, L. Wang, D. Wang, C. Li and J. Jin, Langmuir, 2012, 28, 293–298 CrossRef CAS PubMed.
- J. Kim, L. J. Cote and J. X. Huang, Acc. Chem. Res., 2012, 45, 1356–1364 CrossRef CAS PubMed.
- F. Kim, L. J. Cote and J. Huang, Adv. Mater., 2010, 22, 1954–1958 CrossRef CAS PubMed.
- H. Wang and H. Dai, Chem. Soc. Rev., 2013, 42, 3088–3113 RSC.
- D. Wang, R. Kou, D. Choi, Z. Yang, Z. Nie, J. Li, L. V. Saraf, D. Hu, J. Zhang, G. L. Graff, J. Liu, M. A. Pope and I. A. Aksay, ACS Nano, 2010, 4, 1587–1595 CrossRef CAS PubMed.
- Y. Su, S. Li, D. Wu, F. Zhang, H. Liang, P. Gao, C. Cheng and X. Feng, ACS Nano, 2012, 6, 8349–8356 CrossRef CAS PubMed.
- Q. Qu, S. Yang and X. Feng, Adv. Mater., 2011, 23, 5574–5580 CrossRef CAS PubMed.
- Z. S. Wu, W. Ren, L. Wen, L. Gao, J. Zhao, Z. Chen, G. Zhou, F. Li and H.-M. Cheng, ACS Nano, 2010, 4, 3187–3194 CrossRef CAS PubMed.
- S. Chen, J. Zhu, X. Wu, Q. Han and X. Wang, ACS Nano, 2010, 4, 2822–2830 CrossRef CAS PubMed.
- J. Yan, Z. Fan, W. Sun, G. Ning, T. Wei, Q. Zhang, R. Zhang, L. Zhi and F. Wei, Adv. Funct. Mater., 2012, 22, 2632–2641 CrossRef CAS PubMed.
- S. Chen, J. Zhu and X. Wang, ACS Nano, 2010, 4, 6212–6218 CrossRef CAS PubMed.
- S. Z. Butler, S. M. Hollen, L. Cao, Y. Cui, J. A. Gupta, H. R. Gutiérrez, T. F. Heinz, S. S. Hong, J. Huang, A. F. Ismach, E. Johnston-Halperin, M. Kuno, V. V. Plashnitsa, R. D. Robinson, R. S. Ruoff, S. Salahuddin, J. Shan, L. Shi, M. G. Spencer, M. Terrones, W. Windl and J. E. Goldberger, ACS Nano, 2013, 7, 2898–2926 CrossRef CAS PubMed.
- M. Osada and T. Sasaki, Adv. Mater., 2012, 24, 210–228 CrossRef CAS PubMed.
- R. Ma, Z. Liu, L. Li, N. Iyi and T. Sasaki, J. Mater. Chem., 2006, 16, 3809–3813 RSC.
- Z. P. Liu, R. Z. Ma, M. Osada, N. Iyi, Y. Ebina, K. Takada and T. Sasaki, J. Am. Chem. Soc., 2006, 128, 4872–4880 CrossRef CAS PubMed.
- J. Liang, R. Ma, N. Iyi, Y. Ebina, K. Takada and T. Sasaki, Chem. Mater., 2010, 22, 371–378 CrossRef CAS.
- D. S. Kim, T. C. Ozawa, K. Fukuda, S. Ohshima, I. Nakai and T. Sasaki, Chem. Mater., 2011, 23, 2700–2702 CrossRef CAS.
- Y. Omomo, T. Sasaki, L. Z. Wang and M. Watanabe, J. Am. Chem. Soc., 2003, 125, 3568–3575 CrossRef CAS PubMed.
- J. Feng, X. Sun, C. Wu, L. Peng, C. Lin, S. Hu, J. Yang and Y. Xie, J. Am. Chem. Soc., 2011, 133, 17832–17838 CrossRef CAS PubMed.
- Q. Wang and D. O'Hare, Chem. Rev., 2012, 112, 4124–4155 CrossRef CAS PubMed.
- S. Ida, D. Shiga, M. Koinuma and Y. Matsumoto, J. Am. Chem. Soc., 2008, 130, 14038–14039 CrossRef CAS PubMed.
- C. Nethravathi, B. Viswanath, M. Sebastian and M. Rajamathi, J. Colloid Interface Sci., 2010, 345, 109–115 CrossRef CAS PubMed.
- L. Li, R. Ma, Y. Ebina, K. Fukuda, K. Takada and T. Sasaki, J. Am. Chem. Soc., 2007, 129, 8000–8007 CrossRef CAS PubMed.
- L. Dai, D. W. Chang, J.-B. Baek and W. Lu, Small, 2012, 8, 1130–1166 CrossRef CAS PubMed.
- Z. Chen, D. Zhang, X. Wang, X. Jia, F. Wei, H. Li and Y. Lu, Adv. Mater., 2012, 24, 2030–2036 CrossRef CAS PubMed.
- Z. Chen, V. Augustyn, J. Wen, Y. Zhang, M. Shen, B. Dunn and Y. Lu, Adv. Mater., 2011, 23, 791–795 CrossRef CAS PubMed.
- Z. Chen, V. Augustyn, X. Jia, Q. Xiao, B. Dunn and Y. Lu, ACS Nano, 2012, 6, 4319–4327 CrossRef CAS PubMed.
- H. W. Liang, J. W. Liu, H. S. Qian and S. H. Yu, Acc. Chem. Res., 2013, 46, 1450–1461 CrossRef CAS PubMed.
- X. Wang and Y. Li, J. Am. Chem. Soc., 2002, 124, 2880–2881 CrossRef CAS PubMed.
- S. D. Perera, B. Patel, N. Nijem, K. Roodenko, O. Seitz, J. P. Ferraris, Y. J. Chabal and K. J. Balkus, Jr, Adv. Energy Mater., 2011, 1, 936–945 CrossRef CAS PubMed.
- J. W. Liu, H. W. Liang and S. H. Yu, Chem. Rev., 2012, 112, 4770–4799 CrossRef CAS PubMed.
- P. Chen, J. J. Yang, S. S. Li, Z. Wang, T. Y. Xiao, Y. H. Qian and S. H. Yu, Nano Energy, 2013, 2, 249–256 CrossRef CAS PubMed.
- Y. Xu, Z. Lin, X. Huang, Y. Liu, Y. Huang and X. Duan, ACS Nano, 2013, 7, 4042–4049 CrossRef CAS PubMed.
- J. Chen, K. Sheng, P. Luo, C. Li and G. Shi, Adv. Mater., 2012, 24, 4569–4573 CrossRef CAS PubMed.
- W. Chen and L. Yan, Nanoscale, 2011, 3, 3132–3137 RSC.
- C. Li and G. Shi, Nanoscale, 2012, 4, 5549–5563 RSC.
- Z. S. Wu, Y. Sun, Y. Z. Tan, S. Yang, X. Feng and K. Muellen, J. Am. Chem. Soc., 2012, 134, 19532–19535 CrossRef CAS PubMed.
- A. S. Arico, P. Bruce, B. Scrosati, J. M. Tarascon and W. Van Schalkwijk, Nat. Mater., 2005, 4, 366–377 CrossRef CAS PubMed.
- M. A. Worsley, P. J. Pauzauskie, T. Y. Olson, J. Biener, J. H. Satcher, Jr and T. F. Baumann, J. Am. Chem. Soc., 2010, 132, 14067–14069 CrossRef CAS PubMed.
- S. Nardecchia, D. Carriazo, M. Luisa Ferrer, M. C. Gutierrez and F. del Monte, Chem. Soc. Rev., 2013, 42, 794–830 RSC.
- W. Chen, S. Li, C. Chen and L. Yan, Adv. Mater., 2011, 23, 5679–5683 CrossRef CAS PubMed.
- Z. Zhang, F. Xiao, Y. Guo, S. Wang and Y. Liu, ACS Appl. Mater. Interfaces, 2013, 5, 2227–2233 CAS.
- X. L. Wu, T. Wen, H. L. Guo, S. Yang, X. Wang and A. W. Xu, ACS Nano, 2013, 7, 3589–3597 CrossRef CAS PubMed.
- H. Zhu, X. Wang, F. Yang and X. Yang, Adv. Mater., 2011, 23, 2745–2748 CrossRef CAS PubMed.
- Y. Y. Li, Z. S. Li and P. K. Shen, Adv. Mater., 2013, 25, 2474–2480 CrossRef CAS PubMed.
- Y. H. Lin, T. Y. Wei, H. C. Chien and S. Y. Lu, Adv. Energ. Mater., 2011, 1, 901–907 CrossRef CAS PubMed.
- H. C. Chien, W. Y. Cheng, Y. H. Wang and S. Y. Lu, Adv. Funct. Mater., 2012, 22, 5038–5043 CrossRef CAS PubMed.
- Z. Y. Wang, Z. C. Wang, H. B. Wu and X. W. Lou, Sci. Rep., 2013, 3, 1391 Search PubMed.
- N. Yan, F. Wang, H. Zhong, Y. Li, Y. Wang, L. Hu and Q. W. Chen, Sci. Rep., 2013, 3, 1568 Search PubMed.
- L. Li, K. H. Seng, Z. X. Chen, Z. P. Guo and H. K. Liu, Nanoscale, 2013, 5, 1922–1928 RSC.
- W. Xiao, D. Wang and X. W. Lou, J. Phys. Chem. C, 2009, 114, 1694–1700 Search PubMed.
- X. M. Yin, C. C. Li, M. Zhang, Q. Y. Hao, S. Liu, L. B. Chen and T. H. Wang, J. Phys. Chem. C, 2010, 114, 8084–8088 CAS.
- Q. Qu, Y. Zhu, X. Gao and Y. Wu, Adv. Energy Mater., 2012, 2, 950–955 CrossRef CAS PubMed.
- Z. Dong, X. Lai, J. E. Halpert, N. Yang, L. Yi, J. Zhai, D. Wang, Z. Tang and L. Jiang, Adv. Mater., 2012, 24, 1046–1049 CrossRef CAS PubMed.
- X. Wang, X. L. Wu, Y. G. Guo, Y. Zhong, X. Cao, Y. Ma and J. Yao, Adv. Funct. Mater., 2010, 20, 1680–1686 CrossRef CAS PubMed.
- Z. P. Liu, R. Z. Ma, M. Osada, K. Takada and T. Sasaki, J. Am. Chem. Soc., 2005, 127, 13869–13874 CrossRef CAS PubMed.
- M. Q. Zhao, Q. Zhang, J. Q. Huang and F. Wei, Adv. Funct. Mater., 2012, 22, 675–694 CrossRef CAS PubMed.
- X. H. Liu, R. Z. Ma, Y. Bando and T. Sasaki, Angew. Chem., Int. Ed., 2010, 49, 8253–8256 CrossRef CAS PubMed.
- L. Wang, Z. H. Dong, Z. G. Wang, F. X. Zhang and J. Jin, Adv. Funct. Mater., 2012, 23, 2758–2764 CrossRef PubMed.
- N. Li, G. Zhou, F. Li, L. Wen and H. Cheng, Adv. Funct. Mater., 2013, 23, 5429–5435 CrossRef CAS PubMed.
- K. Park, A. Benayad, D. Kang and S. Doo, J. Am. Chem. Soc., 2008, 130, 14930–14931 CrossRef CAS PubMed.
- Y. Liu, D. Liu, Q. Zhang, D. Yu, J. Liu and G. Cao, Electrochim. Acta, 2011, 56, 2559–2565 CrossRef CAS PubMed.
- S. Amaresh, K. Karthikeyan, K. Kim, J. An, S. Cho, K. Chung, B. Cho, K. Nam and Y. Lee, J. Nanosci. Nanotechnol., 2014, 14, 7545–7552 CrossRef PubMed.
- Y. Liu, Y. Gao, Q. Wang and A. Dou, J. Alloys Compd., 2014, 605, 1–6 CrossRef CAS PubMed.
- C. Zhou, Y. Zhang, Y. Li and J. Liu, Nano Lett., 2013, 13, 2078–2085 CrossRef CAS PubMed.
- L. Hu, F. La Mantia, H. Wu, X. Xie, J. McDonough, M. Pasta and Y. Cui, Adv. Energy Mater., 2011, 1, 1012–1017 CrossRef CAS PubMed.
- B. Liu, J. Zhang, X. Wang, G. Chen, D. Chen, C. Zhou and G. Shen, Nano Lett., 2012, 12, 3005–3011 CrossRef CAS PubMed.
- C. T. Hsieh, W. Y. Chen and Y. S. Cheng, Electrochim. Acta, 2010, 55, 5294–5300 CrossRef CAS PubMed.
- X. Zhang, X. Lu, Y. Shen, J. Han, L. Yuan, L. Gong, Z. Xu, X. Bai, M. Wei, Y. Tong, Y. Gao, J. Chen, J. Zhou and Z. L. Wang, Chem. Commun., 2011, 47, 5804–5806 RSC.
- C. T. Hsieh, H. Teng, W. Y. Chen and Y. S. Cheng, Carbon, 2010, 48, 4219–4229 CrossRef CAS PubMed.
- J. W. Long, B. Dunn, D. R. Rolison and H. S. White, Chem. Rev., 2004, 104, 4463–4492 CrossRef CAS.
- H. Zhang, X. Yu and P. V. Braun, Nat. Nanotechnol., 2011, 6, 277–281 CrossRef CAS PubMed.
- S. R. Gowda, A. L. M. Reddy, X. Zhan, H. R. Jafry and P. M. Ajayan, Nano Lett., 2012, 12, 1198–1202 CrossRef CAS PubMed.
- F. Meng and Y. Ding, Adv. Mater., 2011, 23, 4098–4102 CrossRef CAS PubMed.
- L. Xingyou, A. Hirata, T. Fujita and C. Mingwei, Nat. Nanotechnol., 2011, 6, 232–236 CrossRef PubMed.
- D. Pech, M. Brunet, H. Durou, P. Huang, V. Mochalin, Y. Gogotsi, P. L. Taberna and P. Simon, Nat. Nanotechnol., 2010, 5, 651–654 CrossRef CAS PubMed.
- L. Hu, M. Pasta, F. La Mantia, L. Cui, S. Jeong, H. D. Deshazer, J. W. Choi, S. M. Han and Y. Cui, Nano Lett., 2010, 10, 708–714 CrossRef CAS PubMed.
- L. Hu, W. Chen, X. Xie, N. Liu, Y. Yang, H. Wu, Y. Yao, M. Pasta, H. N. Alshareef and Y. Cui, ACS Nano, 2011, 5, 8904–8913 CrossRef CAS PubMed.
- X. Xie, L. Hu, M. Pasta, G. F. Wells, D. Kong, C. S. Criddle and Y. Cui, Nano Lett., 2011, 11, 291–296 CrossRef CAS PubMed.
- G. Yu, L. Hu, M. Vosgueritchian, H. Wang, X. Xie, J. R. McDonough, X. Cui, Y. Cui and Z. Bao, Nano Lett., 2011, 11, 2905–2911 CrossRef CAS PubMed.
- L. Bao and X. Li, Adv. Mater., 2012, 24, 3246–3252 CrossRef CAS PubMed.
- X. Xie, M. Ye, L. B. Hu, N. Liu, J. R. McDonough, W. Chen, H. N. Alshareef, C. S. Criddle and Y. Cui, Energy Environ. Sci., 2012, 5, 5265–5270 CAS.
- W. Chen, R. B. Rakhi, L. Hu, X. Xie, Y. Cui and H. N. Alshareef, Nano Lett., 2011, 11, 5165–5172 CrossRef CAS PubMed.
- J. Ge, H. B. Yao, W. Hu, X. F. Yu, Y. X. Yan, L. B. Mao, H. H. Li, S. S. Li and S.-H. Yu, Nano Energy, 2013, 2, 505–513 CrossRef CAS PubMed.
- L. Hu and Y. Cui, Energy Environ. Sci., 2012, 5, 6423–6435 Search PubMed.
- L. Y. Yuan, B. Yao, B. Hu, K. F. Huo, W. Chen and J. Zhou, Energy Environ. Sci., 2013, 6, 470–476 CAS.
- L. Hu, J. W. Choi, Y. Yang, S. Jeong, F. La Mantia, L. F. Cui and Y. Cui, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 21490–21494 CrossRef CAS PubMed.
- R. B. Rakhi, W. Chen, D. Cha and H. N. Alshareef, Nano Lett., 2012, 12, 2559–2567 CrossRef CAS PubMed.
- D. Wei, B. Wu, Y. Guo, G. Yu and Y. Liu, Acc. Chem. Res., 2012, 46, 106–115 CrossRef PubMed.
- Y. Ding, M. A. Invernale and G. A. Sotzing, ACS Appl. Mater. Interfaces, 2010, 2, 1588–1593 CAS.
- J. Jiang, J. Liu, R. Ding, J. Zhu, Y. Li, A. Hu, X. Li and X. Huang, ACS Appl. Mater. Interfaces, 2011, 3, 99–103 CAS.
- G. Yu, L. Hu, N. Liu, H. Wang, M. Vosgueritchian, Y. Yang, Y. Cui and Z. Bao, Nano Lett., 2011, 11, 4438–4442 CrossRef CAS PubMed.
- K. Yu Jin, C. Haegeun, H. Chi-Hwan and K. Woong, Nanotechnology, 2012, 23, 65401 CrossRef PubMed.
- Y. J. Kang, S. J. Chun, S. S. Lee, B. Y. Kim, J. H. Kim, H. Chung, S. Y. Lee and W. Kim, ACS Nano, 2012, 6, 6400–6406 CrossRef CAS PubMed.
- Z. Weng, Y. Su, D.-W. Wang, F. Li, J. Du and H.-M. Cheng, Adv. Energy Mater., 2011, 1, 917–922 CrossRef CAS PubMed.
- C. Meng, C. Liu, L. Chen, C. Hu and S. Fan, Nano Lett., 2010, 10, 4025–4031 CrossRef CAS PubMed.
- B. G. Choi, J. Hong, W. H. Hong, P. T. Hammond and H. Park, ACS Nano, 2011, 5, 7205–7213 CrossRef CAS PubMed.
- A. Sumboja, C. Y. Foo, X. Wang and P. S. Lee, Adv. Mater., 2013, 25, 2809–2815 CrossRef CAS PubMed.
- H. C. Gao, F. Xiao, C. B. Ching and H. W. Duan, ACS Appl. Mater. Interfaces, 2012, 4, 7020–7026 CAS.
- C. Chen, Q. H. Yang, Y. Yang, W. Lv, Y. Wen, P. X. Hou, M. Wang and H. M. Cheng, Adv. Mater., 2009, 21, 3007–3011 CrossRef CAS PubMed.
- H. Huang, P. W. Chen, X. T. Zhang, Y. Lu and W. C. Zhan, Small, 2013, 9, 1397–1404 CrossRef CAS PubMed.
- J. Zou, J. Liu, A. S. Karakoti, A. Kumar, D. Joung, Q. Li, S. I. Khondaker, S. Seal and L. Zhai, ACS Nano, 2010, 4, 7293–7302 CrossRef CAS PubMed.
- Y. Zhao, J. Liu, Y. Hu, H. H. Cheng, C. G. Hu, C. C. Jiang, L. Jiang, A. Y. Cao and L. T. Qu, Adv. Mater., 2013, 25, 591–595 CrossRef CAS PubMed.
- Z. S. Wu, S. Yang, Y. Sun, K. Parvez, X. Feng and K. Muellen, J. Am. Chem. Soc., 2012, 134, 9082–9085 CrossRef CAS PubMed.
- X. Chen, H. Zhu, Y.-C. Chen, Y. Shang, A. Cao, L. Hu and G. W. Rubloff, ACS Nano, 2012, 6, 7948–7955 CrossRef CAS PubMed.
- F. Liu, S. Song, D. Xue and H. Zhang, Adv. Mater., 2012, 24, 1089–1094 CrossRef CAS PubMed.
- K. Wang, Q. H. Meng, Y. J. Zhang, Z. X. Wei and M. H. Miao, Adv. Mater., 2013, 25, 1494–1498 CrossRef CAS PubMed.
- Z. Xu, Y. Zhang, P. G. Li and C. Gao, ACS Nano, 2012, 6, 7103–7113 CrossRef CAS PubMed.
- H. P. Cong, X. C. Ren, P. Wang and S. H. Yu, Sci. Rep., 2012, 2, 613 Search PubMed.
- Z. L. Dong, C. C. Jiang, H. H. Cheng, Y. Zhao, G. Q. Shi, L. Jiang and L. T. Qu, Adv. Mater., 2012, 24, 1856–1861 CrossRef CAS PubMed.
- P. Yang, X. Xiao, Y. Li, Y. Ding, P. Qiang, X. Tan, W. Mai, Z. Lin, W. Wu, T. Li, H. Jin, P. Liu, J. Zhou, C. P. Wong and Z. L. Wang, ACS Nano, 2013, 7, 2617–2626 CrossRef CAS PubMed.
- J. Bae, Y. J. Park, M. Lee, S. N. Cha, Y. J. Choi, C. S. Lee, J. M. Kim and Z. L. Wang, Adv. Mater., 2011, 23, 3446–3449 CrossRef CAS PubMed.
- D. Choi, M. Y. Choi, W. M. Choi, H. J. Shin, H. K. Park, J. S. Seo, J. Park, S. M. Yoon, S. J. Chae, Y. H. Lee, S. W. Kim, J. Y. Choi, S. Y. Lee and J. M. Kim, Adv. Mater., 2010, 22, 2187–2192 CrossRef CAS PubMed.
- L. Yuan, X. Xiao, T. Ding, J. Zhong, X. Zhang, Y. Shen, B. Hu, Y. Huang, J. Zhou and Z. L. Wang, Angew. Chem., Int. Ed., 2012, 51, 4934–4938 CrossRef CAS PubMed.
- I. Kovalenko, B. Zdyrko, A. Magasinski, B. Hertzberg, Z. Milicev, R. Burtovyy, I. Luzinov and G. Yushin, Science, 2011, 333, 75–79 CrossRef PubMed.
- S. Sarkar, P. S. Veluri and S. Mitra, Electrochim. Acta, 2014, 132, 448–456 CrossRef CAS PubMed.
- J. Liu, Q. Zhang, Z. Wu, J. Wu, J. Li, L. Huang and S. Sun, Chem. Commun., 2014, 50, 6386–6389 RSC.
- Y. Hou, Y. W. Cheng, T. Hobson and J. Liu, Nano Lett., 2010, 10, 2727–2733 CrossRef CAS PubMed.
- G. Liu, S. Xun, N. Vukmirovic, X. Song, P. Olalde-Velasco, H. Zheng, V. S. Battaglia, L. Wang and W. Yang, Adv. Mater., 2011, 23, 4679–4683 CrossRef CAS PubMed.
- Y. Li, X. Zhao, Q. Xu, Q. Zhang and D. Chen, Langmuir, 2011, 27, 6458–6463 CrossRef CAS PubMed.
- B. Kang and G. Ceder, Nature, 2009, 458, 190–193 CrossRef CAS PubMed.
- G. Wang, X. Lu, Y. Ling, T. Zhai, H. Wang, Y. Tong and Y. Li, ACS Nano, 2012, 6, 10296–10302 CrossRef CAS PubMed.
- W. Lai, C. K. Erdonmez, T. F. Marinis, C. K. Bjune, N. J. Dudney, F. Xu, R. Wartena and Y. M. Chiang, Adv. Mater., 2010, 22, E139–E144 CrossRef CAS PubMed.
- C. J. Bae, C. K. Erdonmez, J. W. Halloran and Y. M. Chiang, Adv. Mater., 2013, 25, 1254–1258 CrossRef CAS PubMed.
- L. Wang, D. Wang, X. Y. Dong, Z. J. Zhang, X. F. Pei, X. J. Chen, B. Chen and J. Jin, Chem. Commun., 2011, 47, 3556–3558 RSC.
- M. Latorre-Sanchez, P. Atienzar, G. Abellan, M. Puche, V. Fornes, A. Ribera and H. Garcia, Carbon, 2012, 50, 518–525 CrossRef CAS PubMed.
- Y. R. Lee, I. Y. Kim, T. W. Kim, J. M. Lee and S.-J. Hwang, Chem.–Eur. J., 2012, 18, 2263–2271 CrossRef CAS PubMed.
- Y. Li and Y. Wu, J. Am. Chem. Soc., 2009, 131, 5851–5857 CrossRef CAS PubMed.
- H. Kang, G. Huang, S. Ma, Y. Bai, H. Ma, Y. Li and X. Yang, J. Phys. Chem. C, 2009, 113, 9157–9163 CAS.
- T. Sun, G. Qing, B. Su and L. Jiang, Chem. Soc. Rev., 2011, 40, 2909–2921 RSC.
- Q. Ye, F. Zhou and W. Liu, Chem. Soc. Rev., 2011, 40, 4244–4258 RSC.
- L. Hu, M. Chen, X. Fang and L. Wu, Chem. Soc. Rev., 2012, 41, 1350–1362 RSC.
- P. C. Chen, G. Shen, Y. Shi, H. Chen and C. Zhou, ACS Nano, 2010, 4, 4403–4411 CrossRef CAS PubMed.
- M. Duduta, B. Ho, V. C. Wood, P. Limthongkul, V. E. Brunini, W. C. Carter and Y. M. Chiang, Adv. Energy Mater., 2011, 1, 511–516 CrossRef CAS PubMed.
- Y. R. Wang, P. He and H. S. Zhou, Adv. Energy Mater., 2012, 2, 770–779 CrossRef CAS PubMed.
- W. Yarong, H. Ping and Z. Haoshen, Adv. Energy Mater., 2012, 2, 770–779 CrossRef PubMed.
- Y. Yang, G. Y. Zheng and Y. Cui, Energy Environ. Sci., 2013, 6, 1552–1558 CAS.
- Z. L. Wang, D. Xu, J. J. Xu, L. L. Zhang and X. B. Zhang, Adv. Funct. Mater., 2012, 22, 3699–3705 CrossRef CAS PubMed.
- M. B. Sassin, C. N. Chervin, D. R. Rolison and J. W. Long, Acc. Chem. Res., 2012, 46, 1062–1074 CrossRef PubMed.
- H. Jiang, C. Li, T. Sun and J. Ma, Chem. Commun., 2012, 48, 2606–2608 RSC.
- F. Cao, G. X. Pan, P. S. Tang and H. F. Chen, J. Power Sources, 2012, 216, 395–399 CrossRef CAS PubMed.
- J. Zhu and J. He, ACS Appl. Mater. Interfaces, 2012, 4, 1770–1776 CAS.
- H. P. Cong, X. C. Ren, P. Wang and S. H. Yu, ACS Nano, 2012, 6, 2693–2703 CrossRef CAS PubMed.
- L. Wei, M. Sevilla, A. B. Fuertes, R. Mokaya and G. Yushin, Adv. Energy Mater., 2011, 1, 356–361 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |


