Synthesis, characterization and cytotoxicity of europium incorporated ZnO–graphene nanocomposites on human MCF7 breast cancer cells†
Abstract
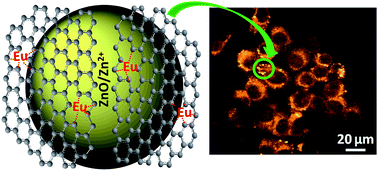
Europium incorporated ZnO-chemically converted graphene (CCG) nanocomposites (ZEG) were synthesized by adopting a solvothermal process at 95 °C from the precursors of varying europium nitrate to zinc acetate molar ratios (R = 0.00, 0.05, 0.10, 0.15) in a fixed content of graphene oxide. Eu level (R value) in the precursors was found to play a role on tailoring the crystallite/particle size of hexagonal ZnO, as evidenced from X-ray diffraction/transmission electron microscopy analysis. The presence of chemical interaction/complexation between the oxygen functionalities of CCG and inorganic moieties (ZnO/Zn2+ and Eu ions) of nanocomposites were studied by FTIR, Raman, UV-Vis spectral and XPS measurements. The nanocomposites possessed meso pores as confirmed from BET nitrogen adsorption isotherm, and the sample ZEG(10) (R = 0.10) was found to possess the highest specific surface area. In spite of an UV emission at ∼385 nm, an orange emission (appeared at 595 nm) along with other visible emissions was observed from the photoluminescence spectra of nanocomposites. However, the intensity of the orange emission (λex = 400 nm) was found to be maximum in the ZEG(10) sample, which produced relatively bright orange fluorescent images of human breast cancer cells (MCF7) under confocal laser scanning microscope. This indicated the internalization of the nanomaterials within the cells. As obtained from the MTT assay, the samples (R ≥ 0.10) exhibited comparatively low in vitro cytotoxicity (higher cell viability) on the cancer cells. The low cytotoxicity could be explained on the basis of the ID/IG (intensities of D and G bands of graphene) value of CCG, as realized from the Raman spectral measurement. The nanocomposite ZEG(10), having relatively large surface area, bright cell imaging capability and better cell viability, could be employed for cancer cell targeted optical imaging and drug delivery.

 Please wait while we load your content...
Please wait while we load your content...