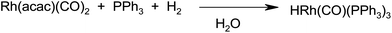Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
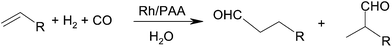
“On water” hydroformylation of 1-hexene using Rh/PAA (PAA = polyacrylic acid) as catalyst
W.
Alsalahi
and
A. M.
Trzeciak
*
Faculty of Chemistry, University of Wrocław, 14 F. Joliot-Curie St., 50-383 Wrocław, Poland. E-mail: anna.trzeciak@chem.uni.wroc.pl
First published on 1st July 2014
Abstract
A new rhodium catalyst, Rh/PAA, obtained by the immobilization of Rh(acac)(CO)2 on polyacrylic acid (PAA), was successfully applied for the hydroformylation of 1-hexene in a water medium. Spectroscopic analysis evidenced that rhodium in Rh/PAA was chemically bonded to polyacrylic acid and formed a hydrido-carbonyl rhodium compound in reaction with H2/CO. Excellent results (98% conversion, TOF 1000) were obtained in the “on water” hydroformylation of 1-hexene when Rh/PAA was used together with a hydrophobic phosphine (triphenylphosphine, tri-p-tolylphosphine, or diphenyl(2-methoxyphenyl) phosphine). A similar efficiency was also obtained for a system composed of Rh(acac)(CO)2 and PPh3, tested in the same conditions in water.
1. Introduction
The hydroformylation reaction of olefins is a very important industrial process used for the production of aldehydes.1 This reaction was discovered in 1938 by Otto Roelen for a cobalt complex at Ruhrchemie in Germany.1 The aqueous–organic biphasic process for propene and butene hydroformylation was commercialized at Ruhrchemie-Rhone Poulenc in 1984. The main advantage offered by this process is the efficient separation of the catalyst from the organic products. This is possible because the catalyst bearing a water-soluble phosphine is kept in the aqueous phase, which is not miscible with the organic phase containing aldehydes.1Water is the most plentiful, non-environmentally harmful, nontoxic, and nonflammable solvent used by nature for biological transformations, very attractive from an economical point of view. However, water also possesses the extraordinary ability to catalyze chemical transformations between some insoluble organic reactants.2–6 Moreover, unique reactivity and selectivity is observed in water when compared with conventional organic solvents.7–10 In 1980, Rideout and Breslow discovered rate acceleration of Diels–Alder reactions between nonpolar compounds in homogeneous aqueous solutions compared with the same reactions in organic solvents.11 In 2005, Sharpless and his co-workers reported examples of organic syntheses performed in a water medium with reactants insoluble in water.9 Those reactions, termed “on water”, gave higher yields and faster kinetics than in any organic solvent. An on-water reaction is the chemical process that takes place at the organic/water phase boundary (emulsion). The formation of hydrogen bonds in interfacial water molecules at the hydrophobic interface has a free dangling hydroxyl group that protrudes into the organic phase, plays a key role in catalyzing reactions, and demonstrates an extraordinary reaction rate acceleration.9,12 Such interactions were also studied in catalytic systems for hydroformylation.13–15
In this paper, we report a new catalytic system for an on-water hydroformylation process by applying of a water-soluble immobilized catalyst, Rh/PAA, and a hydrophobic phosphine. The second system studied in the same conditions contained a water-insoluble complex, Rh(acac)(CO)2, as the rhodium source. These systems, applied for the first time in the hydroformylation of 1-hexene in water, gave very promising results. Until now there has been no detailed reports on the hydroformylation of higher olefins using the on-water methodology.
2. Experimental
2.1. Materials
The rhodium complex Rh(acac)(CO)2 was prepared according to the literature.16Polyacrylic acids (Mw ∼ 1800 and 450![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) were purchased from Sigma-Aldrich; triphenylphosphine (PPh3) was purchased from Avocado; 1-hexene was purchased from Merck; 1-octene was purchased from Sigma-Aldrich; hydrogen (H2, 99.999%) and carbon monoxide (CO, 99.97%) were procured from Air Products. All chemicals were used without any additional purification. Distilled water was used as the reaction medium.
000) were purchased from Sigma-Aldrich; triphenylphosphine (PPh3) was purchased from Avocado; 1-hexene was purchased from Merck; 1-octene was purchased from Sigma-Aldrich; hydrogen (H2, 99.999%) and carbon monoxide (CO, 99.97%) were procured from Air Products. All chemicals were used without any additional purification. Distilled water was used as the reaction medium.
2.2. Synthesis of Rh/PAA (Mw ∼ 1800)17
A solution of PAA (Mw ∼ 1800, 1.5 g) dissolved in water (30 ml) was added to a suspension of Rh(acac)(CO)2 (1 g) in water (20 ml). After stirring for 24 h at room temperature, a second portion of PAA (1.5 g) in water (20 ml) was added and stirring continued for the next 24 h. During that time, the color of the mixture changed from green to black. Next, the mixture was filtered off, and the resulting filtrate was evaporated using a rotary evaporator yielding a black film of Rh/PAA containing 5.4% of Rh. A second synthesis, performed according to the same procedure but at the temperature of 35 °C, gave a product containing 10.2% of Rh.2.3. Synthesis of Rh/PAA (Mw ∼ 450![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) 000)
000)
A solution of PAA (Mw ∼ 450![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) (1.5 g) in water (30 ml) was added to a suspension of Rh(acac)(CO)2 (1 g) in water (20 ml), and the mixture was stirred for 24 h at room temperature. The color changed from green to black. The mixture was filtered off, and the resulting filtrate was evaporated using a rotary evaporator yielding a black film of Rh/PAA containing 0.1% of Rh.
000) (1.5 g) in water (30 ml) was added to a suspension of Rh(acac)(CO)2 (1 g) in water (20 ml), and the mixture was stirred for 24 h at room temperature. The color changed from green to black. The mixture was filtered off, and the resulting filtrate was evaporated using a rotary evaporator yielding a black film of Rh/PAA containing 0.1% of Rh.
2.4. Synthesis of HRh(CO)(PPh3)3
A mixture of Rh(acac)(CO)2 (0.00762 g) and PPh3 (0.05136 g) in water (2 ml) was stirred under hydrogen gas (1 bar) at 38 °C for ca. 24 h. The yellow precipitate was filtered off and dried.31P NMR (C6D6): δ 40.60 ppm (d, JRh–P = 151.75 Hz). IR: νRh–H = 2036 cm−1, νCO = 1921 cm−1.
2.5. Preparation of samples for ICP measurements
A 0.03 g sample of Rh/PAA was placed in a 25 ml volumetric flask, and 0.5 ml of concentrated nitric acid and 1.5 of concentrated hydrochloric acid were added. The flask was filled to 25 ml with distilled water, and the contents were mixed until complete dissolution.2.6. Characterization techniques
The IR spectra of the samples were recorded in the range from 400 to 4000 cm−1 with a Bruker Vector 22 IR spectrometer. NMR spectra were recorded at room temperature on a Bruker Avance 500 MHz spectrometer. Transition electron microscopy (TEM) was performed at 200 kV using a FEI Tecnai 20 X-TWIN electron microscope. The Rh/PAA samples were dispersed in water and then suspended on a carbon grid. The Rh content in the Rh/PAA catalyst was determined using inductively coupled plasma atomic emission spectroscopy (ICP-AES) analysis (ARL Model 3410, Fisons Instruments). The reaction products were identified and analyzed with a Hewlett-Packard 5890 II gas chromatograph equipped with an HP-5 column (25 m × 0.2 mm) using helium as the carrier gas. Mass spectra were obtained using an HP 5971A mass selective detector.2.7. Hydroformylation of 1-hexene
Hydroformylation experiments were carried out with 100, 50, and 35 ml stainless steel autoclaves provided with manometers, thermostats, and magnetic stirrers. Weighted samples of Rh/PAA and PPh3 were placed in an autoclave under a nitrogen atmosphere, and the solvent (toluene, methanol, or water) and 1-hexene were introduced. The autoclave was closed, filled with hydrogen (5 bar) 3 times, and then pressurized with synthesis gas (H2–CO = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to 10 bar and heated to 80 °C. After the reaction was finished, the autoclave was cooled to room temperature and depressurized. The organic products were separated by a vacuum transfer procedure and analyzed by means GC and GC-MS.
1) to 10 bar and heated to 80 °C. After the reaction was finished, the autoclave was cooled to room temperature and depressurized. The organic products were separated by a vacuum transfer procedure and analyzed by means GC and GC-MS.
2.8. Turnover frequency (TOF)
The turnover number (TON) is defined as the number of moles of substrate that a mole of the catalyst can convert before becoming inactivated. The turnover frequency (TOF) is defined as the number of molecules reacting per active site in unit time. The TOF values were calculated as moles of the aldehyde [mol of catalyst]−1t−1; t = time (h) was estimated from the linear part of the graph presenting pressure vs. time.3. Results and discussion
3.1. Characterization of Rh/PAA by IR
The IR spectrum of Rh(acac)(CO)2 presented two peaks of carbonyl stretching frequencies (ν(CO)) at 2006 and 2064 cm−1 and two peaks originating from a coordinated acac ligand at 1253 and 1559 cm−1. The IR spectrum of Rh/PAA showed only one ν(CO) peak at 2064 cm−1 and no peaks for acac, indicating its removal from the coordination sphere of rhodium during reaction with PAA. In order to recognize reactivity towards substrates of hydroformylation, a solid sample of Rh/PAA was treated with H2 (5 bar), CO (5 bar), and H2 + CO (10 bar). The reaction with H2 was also performed in benzene. The IR spectra of Rh/PAA, measured after treatment with H2 or CO, showed unchanged bands in the region of ca. 2000 cm−1. In contrast, the IR spectrum of Rh/PAA after reaction with syngas (H2–CO, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) showed two new bands at 2100 and 2000 cm−1, attributed to rhodium-hydride and carbonyl vibrations (Table 1). Moreover, the shifting of ν(CO) from 2064 to 2073 cm−1 was observed (Table 1).
1) showed two new bands at 2100 and 2000 cm−1, attributed to rhodium-hydride and carbonyl vibrations (Table 1). Moreover, the shifting of ν(CO) from 2064 to 2073 cm−1 was observed (Table 1).
| Sample | ν CO | ν CO or νRh-H | ν CO(acac), νCO(PAA), νCH(PAA) | |||
|---|---|---|---|---|---|---|
| Rh(acac)(CO)2 | 2006vs, 2064vs | 1526vs, 1559s | ||||
| PAA | 1714vs | 1454w | 1415w | 1269m, 1165m | ||
| Rh/PAA | 2064w | 1714vs | 1455w | 1412w | 1271m, 1175m | |
| Rh/PAA + H2 | 2064w | 1714vs | 1455w | 1415w | 1272m, 1177m | |
| Rh/PAA + CO | 2065w | 1718s | 1457w | 1405w | 1263m, 1172m | |
| Rh/PAA + H2 + CO (4h) | 2069w | 2000vw, 2100vw | 1718s | 1453w | 1413w | 1262m, 1176m |
| Rh/PAA + H2 + CO (6h) | 2073w | 2006vw, 2104vw | 1711s | 1453w | 1413w | 1269m, 1176m |
| Rh/PAA + H2 + CO + water | 2080m | 2023vw, 1983vw | 1713s | 1454m | 1416w | 1262m, 1171m |
| Rh/PAA + H2 + benzene | 2070w | 1711vs | 1457w | 1415w | 1262m, 1172m | |
The TEM analysis of Rh/PAA did not show any Rh(0) nanoparticles; however, nanoparticles appeared after reaction of Rh/PAA with H2/CO. Small, ca. 4 nm, as well as aggregated Rh(0) nanoparticles were found in different places of the TEM grids (Fig. 1). A quite different result was obtained when Rh/PAA reacted with H2/CO in water. The size of Rh(0) nanoparticles was smaller, about 2 nm.
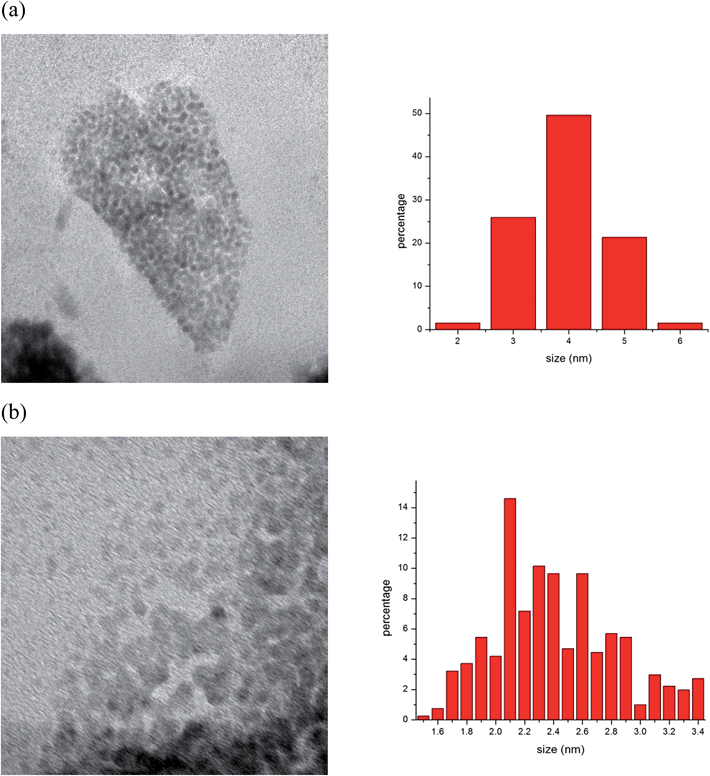 | ||
| Fig. 1 Morphology and rhodium nanoparticle size after reaction of Rh/PAA with: (a) syngas (H2–CO), 10 bar, 80 °C and (b) syngas (H2–CO), 10 bar, 80 °C in water. | ||
3.2. Hydroformylation of 1-hexene
The hydroformylation of 1-hexene was investigated in different solvents with Rh/PAA as the catalyst and a 13-fold excess of a water-insoluble phosphine (PPh3). Aldehydes were formed as the main reaction products, namely a linear aldehyde (1-heptanal) and a branched aldehyde (2-methyl-hexanal) (Scheme 1). Small amounts of the isomerization product, 2-hexene (1–4%), were also found. In some experiments, performed in methanol, traces of an acetalization product, 1,1-dimethoxy-heptane, were identified.In reactions performed without a solvent, in neat 1-hexene, only traces of aldehydes were formed. Similarly, a very low conversion of 1-hexene was noted in toluene, although PPh3 and 1-hexene are soluble in that solvent. Much better results were obtained in methanol and in a methanol–water mixture. In particular, the l/b ratio increased to a good value, 7.3. The addition of methanol to toluene resulted in a significant increase in 1-hexene conversion to aldehydes. Interestingly, when a toluene–water mixture was used, both conversion and selectivity were even better than for a toluene–methanol mixture. Such an effect was surprising because 1-hexene, Rh/PAA, and PPh3 are soluble in methanol and only Rh/PAA is soluble in water. As a rule, a faster reaction is expected in a homogeneous system than in a multiphase one. However, the most spectacular results, in respect to conversion, selectivity, and the reaction rate, were obtained in water only (Table 2). It is worth noting that the volume of water influences the reaction course only very slightly, and almost the same results were obtained using 0.5 or 1 ml of water.
| Solvent | Conversion (%) | 2-Hexene (%) | Aldehydes | l/b | TOF, h−1 | |
|---|---|---|---|---|---|---|
| l (%) | b (%) | |||||
a Reaction conditions: 1-hexene 1.5 ml (0.012 mol), [1-hexene]/[Rh] = 800, [PPh3]/[Rh] = 13, T = 80 °C, H2–CO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) = 10 bar, time = 1 h, autoclave 50 ml.
b Time = 2 h. 1) = 10 bar, time = 1 h, autoclave 50 ml.
b Time = 2 h.
|
||||||
| — | 0.6 | 0.2 | 0.3 | 0.1 | ||
| Toluene (1.5 ml) | 3 | 0.6 | 1.7 | 0.6 | 2.8 | |
Toluene–MeOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 ml)b 0.5 ml)b |
77 | 2 | 59 | 15 | 3.9 | 296 |
Toluene–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 ml) 0.5 ml) |
96 | 3 | 76 | 16 | 4.8 | 940 |
| MeOH (1.5 ml)b | 92 | 4 | 72 | 11 | 6.5 | 530 |
| H2O (0.5 ml) | 96 | 3 | 80 | 12 | 6.7 | 877 |
| H2O (1 ml) | 97 | 3 | 79 | 14 | 5.6 | 849 |
MeOH–H2O (0.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.75 ml) 0.75 ml) |
96 | 4 | 73 | 10 | 7.3 | 827 |
Consequently, further experiments were performed using water as the reaction medium. Such a procedure can be named “on water” because 1-hexene and PPh3 are insoluble in water. The effect of temperature on the hydroformylation of 1-hexene using the catalytic system Rh/PAA + PPh3 was studied at 50–90 °C and a constant pressure of 10 bar (Table 3). An increase in 1-hexene conversion was observed with an increase in temperature. Thus, when temperature increased from 50 to 80 °C, the yield of aldehydes increased from 21 to 94% with a decrease in the reaction time from 3 h to 1 h and a slight increase in the l/b ratio from 4.3 to 6.9. At 90 °C, the yield of aldehydes decreases to 87%, and 6% of 2-hexene, an isomerization product, was formed. Consequently, aldehyde formation was preferred at 80 °C.
| T °C | P, bar | t, min | Conversion (%) | 2-Hexene (%) | Aldehydes | l/b | TOF, h−1 | |
|---|---|---|---|---|---|---|---|---|
| l (%) | b(%) | |||||||
| a Reaction conditions: 1-hexene 1.5 ml (0.012 mol), water 1.5 ml, [Rh] (1.5 × 10−5 mol), [1-hexene]/[Rh] = 800, [PPh3]/[Rh] = 13, autoclave 50 ml. b Autoclave 100 ml, 1-hexene 3 ml, water 3 ml. | ||||||||
| 50 | 10 | 180 | 24 | 1 | 17 | 4 | 4.3 | 56 |
| 60 | 10 | 180 | 65 | 2 | 52 | 11 | 4.7 | 168 |
| 70 | 10 | 60 | 70 | 3 | 56 | 11 | 5.1 | 536 |
| 80 | 10 | 60 | 97 | 3 | 79 | 15 | 5.3 | 803 |
| 90 | 10 | 60 | 98 | 6 | 76 | 11 | 6.9 | 1003 |
| 80 | 8 | 60 | 83 | 7 | 68 | 7 | 9.7 | 677 |
| 80 | 6 | 60 | 62 | 6 | 53 | 3 | 17.7 | 436 |
| 80 | 4 | 62 | 48 | 8 | 38 | 1.7 | 22.2 | 287 |
| 80b | 2 | 240 | 37 | 6 | 30 | 1 | 30 | 63 |
The effect of pressure on the catalytic activity of a Rh/PAA + PPh3 system was studied at 2–10 bar and at 80 °C, and the achieved results are shown in Table 3. When the pressure of the synthesis gas decreased from 10 to 2 bar, the conversion of 1-hexene to the products decreased from 97 to 37% with a decrease in aldehyde yield from 94 to 31%. However, lowering the synthesis gas pressure caused a remarkable increase in the l/b ratio from 5.3 to 30. This is in agreement with earlier observations that a good linearity of aldehydes is favored at low pressure.1 Lower pressure favors the formation of a linear alkyl rhodium species which is next transformed to a linear acyl intermediate and finally to a normal aldehyde.
As can be deduced from the data presented on Fig. 2, the effect of temperature on hydroformylation selectivity is rather small. For instance, the l/b value changed from 4.3 to 6.9 when the temperature rose from 50 to 90 °C. In contrast, the pressure has a decisive effect on the yield of aldehydes as well as on hydroformylation selectivity. At 4 bar, the l/b ratio was 22.2, and at 2 bar, it reached 30.
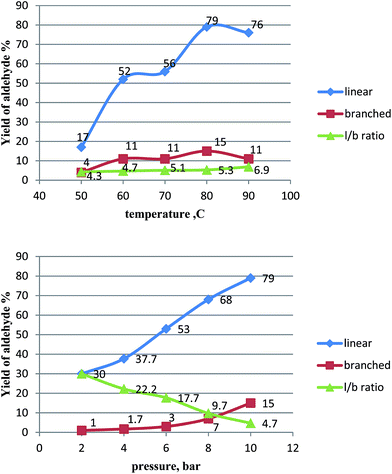 | ||
Fig. 2 Graphs depicting the influence of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CO–H2 pressure and temperature on the selectivity of 1-hexene hydroformylation catalyzed by Rh/PAA + PPh3; [PPh3]/[Rh] = 13, [1-hexene]/[Rh] = 800. 1 CO–H2 pressure and temperature on the selectivity of 1-hexene hydroformylation catalyzed by Rh/PAA + PPh3; [PPh3]/[Rh] = 13, [1-hexene]/[Rh] = 800. | ||
The effect of the [PPh3]/[Rh] ratio on the reaction course was studied in the range from 0 to 13 at 80 °C at 10 bar. The results presented in Table 4 show that in the absence of phosphine Rh/PAA catalyzes only the isomerization of 1-hexene to 2-hexene. However, already a 5-fold excess of PPh3 made it possible to get 57% of aldehydes. With an increase in the [PPh3]/[Rh] ratio, the conversion of 1-hexene increased to 92% together with an increase in the l/b ratio to 5.7. Interestingly, high catalytic activity and good selectivity towards aldehydes were also obtained with the application of other water-insoluble phosphines, namely tri-p-tolylphosphine and diphenyl(2-methoxyphenyl) phosphine with Rh/PAA (Table 4). However, it should be noted that the kind of phosphine has a remarkable influence on the TOF values, and the highest TOF was obtained for PPh3.
| Entry | [L]/[Rh] | Time (min) | Conversion (%) | 2-Hexene (%) | Aldehydes | l/b | TOF, h−1 | |
|---|---|---|---|---|---|---|---|---|
| l (%) | b (%) | |||||||
a L = PPh3.
b L = tri-p-tolylphosphine.
c L = diphenyl(2-methoxyphenyl)phosphine.
d Reaction conditions: 1-hexene 1 ml (0.008 mol), water 1 ml, [Rh] 1 × 10−5 mol, [1-hexene]/[Rh] = 800, 80 °C, H2–CO(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) = 10 bar, autoclave 35 ml. 1) = 10 bar, autoclave 35 ml.
|
||||||||
| 1a | 0 | 240 | 8 | 8 | ||||
| 2a | 5 | 240 | 62 | 5 | 44 | 13 | 3.4 | 86 |
| 3a | 10 | 174 | 90 | 4 | 70 | 16 | 4.4 | 382 |
| 4a | 13 | 120 | 92 | 4 | 88 | 15 | 5.7 | 718 |
| 5b | 13 | 120 | 90 | 4 | 66 | 20 | 3.2 | 538 |
| 6c | 13 | 120 | 85 | 2 | 62 | 20 | 3.1 | 344 |
The hydroformylation of 1-hexene was also performed with another Rh/PAA catalyst containing polyacrylic acid, Mw = 450![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. The rhodium content in this catalyst was significantly lower than in Rh/PAA immobilized on a polymer of Mw = 1800. Nevertheless, this catalysts also gave quite good results, 97% of aldehydes with l/b = 4.6 after 150 min.
000. The rhodium content in this catalyst was significantly lower than in Rh/PAA immobilized on a polymer of Mw = 1800. Nevertheless, this catalysts also gave quite good results, 97% of aldehydes with l/b = 4.6 after 150 min.
In order to recognize the possibility of the in situ formation of an Rh/PAA catalyst, Rh(acac)(CO)2 and PPh3 were used in the next experiment together with PAA added to the reaction mixture. The reaction was even faster than with Rh/PAA; however, a similar yield of the products was obtained. Next, Rh(acac)(CO)2 was used with PPh3 only, without PAA, and, surprisingly, the catalytic result was very promising (Table 5). The final composition of the products was similar to that obtained with Rh/PAA, but the TOF values were higher. At a lower pressure, 4 or 6 bar, l/b increased to attractive values, 16 and 21. Interestingly, the hydrophobic complex HRh(CO)(PPh3)3 can also be successfully applied for the hydroformylation of 1-hexene in water giving the highest TOF, 1903 h−1, noted in these studies.
| Catalyst precursor | P bar | t min | Conversion (%) | 2-Hexene (%) | Aldehydes | l/b | TOF, h−1 | |
|---|---|---|---|---|---|---|---|---|
| l (%) | b (%) | |||||||
| a Reaction conditions: a,d 1-hexene 1 ml (0.008 mol), water 1 ml, autoclave 35 ml, b,c 1-hexene 1.5 ml (0.012 mol), water 1.5 ml, autoclave 50 ml, a,b,c [1-hexene]/[Rh] = 800, [PPh3]/[Rh] = 13, d [1-hexene]/[Rh] = 420, [PPh3]/[Rh] = 6.8. T = 80 °C. | ||||||||
Rh/PAA (Mw ∼ 450![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000)a 000)a |
10 | 150 | 97 | 5 | 74 | 16 | 4.6 | 495 |
| Rh(acac)(CO)2b | 10 | 40 | 97 | 11 | 77 | 5 | 15.4 | 1658 |
| Rh(acac)(CO)2b | 6 | 40 | 76 | 18 | 51 | 2 | 25.2 | 967 |
| Rh(acac)(CO)2b | 4 | 40 | 62 | 20 | 37 | 1 | 37 | 574 |
| Rh(acac)(CO)2d | 10 | 65 | 99 | 4 | 78 | 16 | 4.9 | 1153 |
| Rh(acac)(CO)2d | 6 | 70 | 73.2 | 13 | 56.7 | 3.5 | 16 | 967 |
| Rh(acac)(CO)2d | 4 | 75 | 55.4 | 16.1 | 37.5 | 1.8 | 21 | 374 |
| HRh(CO)(PPh3)3c | 10 | 60 | 98 | 8 | 82 | 6 | 13.7 | 1903 |
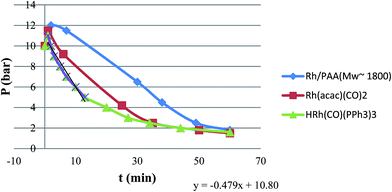
Fig. 3 shows the H2/CO pressure drop during the hydroformylation of 1-hexene catalyzed by Rh/PAA, Rh(acac)(CO)2, and HRh(CO)(PPh3)3. As the last two systems are totally hydrophobic, the name “on water” is suitable for their description.
With these improved reaction conditions in hand, we decided to investigate the on-water hydroformylation of other alkenes. 1-Octene showed good conversion, 98% (Table 6, entry 1). The on-water hydroformylation reaction using 2-hexene and styrene as the substrates gave 76% and 67.0% conversion and 76% and 64% aldehyde selectivity. However, the l/b ratio was expectedly poor, 0.07 and 0.7, respectively (Table 6).
| Substrate | t, min | Conv.a (%) | Aldehydes | l/b | TOF, h−1 | |
|---|---|---|---|---|---|---|
| l (%) | b (%) | |||||
a Rh(acac)(CO)2 as catalyst, [1-hexene]/[Rh] = 420, [PPh3]/[Rh] = 6.5, p = 1 bar.
b Reaction conditions: Substrate 1.5 ml, water 1.5 ml, [sub]/[Rh] = 800, L = PPh3 [PPh3]/[Rh] = 13, T = 80 °C, P(H2–CO) = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, p = 10 bar. 1, p = 10 bar.
|
||||||
| 1-Octene | 120 | 98 | 77 | 13 | 5.9 | 760 |
| 2-Hexene | 180 | 76 | 5 | 71 | 0.07 | 216 |
| Styrene | 90 | 67 | 26 | 38 | 0.7 | 389 |
3.3. Recycling of the catalyst
The reusability of Rh/PAA in the on-water hydroformylation of 1-hexene and 1-octene was studied according to two different protocols. When the reaction with 1-hexene was completed, the organic phase was separated from the aqueous phase by decantation, and the aqueous phase was placed again in an autoclave. All liquid components were removed from the organic phase by “vacuum transfer”, a new portion of olefin and PPh3 were added to the residue, and the resulting mixture was introduced to the autoclave. Thus, in such recycling, practically the entire amount of rhodium was used in the next catalytic cycle. The results presented in Table 7 indicate that recycling was efficient, although the third reaction was slower than the second one. An increase in the l/b ratio from 6.5 to 8.1 was noted.| Substrate | Time min | Conversion (%) | 2-Hexene (%) | Aldehydes % | l/b | TOF, h−1 |
|---|---|---|---|---|---|---|
a Reaction conditions: [sub]/[Rh] = 800, L is PPh3 [PPh3]/[Rh] = 13, T = 80 °C, p(H2–CO) = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, p = 10 bar. 1, p = 10 bar.
|
||||||
| 1-Hexene | 70 | 95.7 | 4.8 | 90.9 | 6.5 | 752 |
| 120 | 95.6 | 4.1 | 90.1 | 6.7 | 751 | |
| 180 | 96.3 | 4.5 | 91.1 | 8.1 | 306 | |
| 1-Octene | 120 | 97.7 | 3.7 | 92.4 | 3.8 | |
| 120 | 77 | 2.4 | 70.9 | 4.1 | ||
| 120 | 95.2 | 2.2 | 91.4 | 3.9 | ||
In reaction with 1-octene as the substrate, only the water phase was used for the first recycling producing 70.9% of aldehydes. For the next run, all rhodium was used again, according to the procedure described for 1-hexene.
3.4. The nature of the catalyst
The results of recycling experiments indicated the leaching of rhodium from the water to the organic phase. To verify that conclusion, the 31P NMR spectrum of the organic phase separated after hydroformylation with Rh/PAA + PPh3 was measured. It showed a broad doublet at 32.2 ppm with JRh–P 133.8 Hz confirming the presence of a rhodium species. In addition, according to the ICP analysis, ca. 50% of rhodium was transferred to the organic phase during the catalytic process.For a better understanding of rhodium transformations in the studied system, the reaction of Rh(acac)(CO)2 with PPh3 and H2 (1 bar) was performed in water. It should be mentioned that PPh3 reacts with Rh(acac)(CO)2 in any organic solvent producing Rh(acac)(CO)(PPh3), which in the presence of a PPh3 excess and H2/CO forms a catalytically active hydrido-carbonyl species. Reaction with H2/CO takes place only under elevated pressure, and no reaction was observed at atmospheric pressure in benzene or toluene. In contrast, formation of HRh(CO)(PPh3)3 was observed in the presence of methanol or isopropanol. Unexpectedly, when a suspension of Rh(acac)(CO)2 and PPh3 in water was treated with H2 (1 bar), the complex HRh(CO)(PPh3)3 was formed in a stoichiometric amount (Scheme 2).
Such a synthetic procedure differs remarkably from those reported in the literature.18–20 The most efficient syntheses of HRh(CO)(PPh3)3 apply KOH, NaBH4, or hydrazine in an ethanol solution.18
4. Conclusions
It was demonstrated that the hydroformylation of 1-hexene can be very efficiently performed in water using water-soluble Rh/PAA or water insoluble Rh(acac)(CO)2 complexes with an excess of hydrophobic PPh3. Such “on water” methodology is very simple and, in particular, the application of an expensive water soluble phosphine is not needed to perform hydroformylation. Also, the organic solvent is eliminated from the system. Moreover, aldehydes were formed as the main products under relatively mild conditions, 80 °C and 10 bar. At a lower pressure, a linear aldehyde can be obtained with excellent selectivity.The presented systems can be used for any higher olefin, as was demonstrated for 1-hexene, 2-hexene, and 1-octene.
Recycling experiments showed that a rhodium catalyst can be recovered without a loss of its catalytic activity. However, during hydroformylation rhodium is leached to the organic phase, which complicates the efficient separation of aldehydes from the catalyst. We are now working on the improvement of this step and preliminary results show that after the catalytic cycle rhodium can be transferred back to the water phase.
Explanation of the high catalytic activity of the presented “on water” system can only in part be based on specific interactions between the reactants, similarly as it was described in the literature. In our opinion, reactions of the rhodium precursor with H2/CO are remarkably influenced by the presence of water, which is not only a solvent but also a reactant. As was shown, in a water medium, a catalytically active HRh(CO)(PPh3)3 complex was formed in high yield under very mild conditions as a result of efficient H2 activation. Such activation was realized without any additives, in particular in the absence of a base that could eventually facilitate heterolytic splitting of H2.
In conclusion it should be pointed out that in the presented catalytic systems water plays not only the role of a reaction medium but it is also involved in the chemical transformations of rhodium complexes to catalytically active forms. A combination of these two functions results in the creation of a very active, simple, and environmentally friendly catalytic system for the hydroformylation of higher olefins.
Acknowledgements
Authors thank Prof. Yuri Varshavsky for important contribution in the synthesis of Rh/PAA and prof. David Cole–Hamilton for very inspiring discussions.References
- (a) Applied Homogenous Catalysis with Organometallic Compounds: A Comprehensive Handbook in Two Volumes, ed. B. Cornils and W. A. Herrmann, VCH Weinheim, 1996 Search PubMed; (b) Rhodium Catalysed Hydroformylation, ed. P. W. N. M. van Leeuwen and C. Claver, Kluwer Academic Publisher, Dordrecht, 2000 Search PubMed; (c) W. G. Parshall and S. D. Ittel, Homogenous Catalysis: The Application and Chemistry of Catalysis by Soluble Transition Metal Complexes, John Wiley & Sons, Inc., New York, 1992 Search PubMed; (d) Metal Catalysis in Industrial Organic Processes, ed. G. P. Chiusoli and P. M. Maitlis, PSC Publishing, 2006 Search PubMed; (e) S. Bhaduri and D. Mukesh, Homogeneous Catalysis. Mechanisms and Industrial Application, Wiley-Interscience, 2000 Search PubMed; (f) Transition Metals for Organic Synthesis. Building Blocks and Fine Chemicals, ed. M. Beller and C. Bolm, Wiley-VCH Verlag GmbH & Co. KGaA, 2004 Search PubMed; (g) M. Beller, B. Cornils, C. D. Frohning and C. W. Kohlpaintner, J. Mol. Catal. A: Chem., 1995, 104, 17 CrossRef CAS; (h) A. M. Trzeciak and J. J. Ziółkowski, Coord. Chem. Rev., 1999, 190–192, 883 CrossRef CAS; (i) A. M. Trzeciak, Hydroformylation in: Comprehensive Inorganic Chemistry II: from elements to applications, ed. J. Reedijk and K. Poeppelmeier, Elsevier, Amsterdam, 2013, vol. 6, p. 25 Search PubMed; (j) B. Breit and W. Seiche, Synthesis, 2001, 1 CrossRef CAS PubMed; (k) A. Behr and P. Neubert, Applied Homogeneous Catalysis, Wiley-VCH, Weinheim, 2012 Search PubMed.
- R. N. Butler and A. G. Coyne, Chem. Rev., 2010, 110, 6302 CrossRef CAS PubMed.
- N. Shapiro and A. Vigalok, Angew. Chem., 2008, 120, 2891 CrossRef.
- S. Mellouli, L. Bousekkine, A. B. Theberge and W. T. S. Huck, Angew. Chem., Int. Ed., 2012, 51, 7981 CrossRef CAS PubMed.
- S. K. Rout, S. Guin, J. Nath and B. K. Patel, Green Chem., 2012, 14, 2491 RSC.
- P. Norcott, C. Spielman and C. S. P. McErlean, Green Chem., 2012, 14, 605 RSC.
- Y. Jung and R. A. Marcus, J. Am. Chem. Soc., 2007, 129(17), 5492 CrossRef CAS PubMed.
- J. S. Yadav, T. Swamy, B. V. S. Reddy and D. K. Rao, J. Mol. Catal. A: Chem., 2007, 274, 116 CrossRef CAS PubMed.
- S. Narayan, J. Muldoon, M. G. Finn, V. V. Fokin, H. C. Kolb and K. B. Sharpless, Angew. Chem., Int. Ed., 2005, 44, 3275 CrossRef CAS PubMed.
- F. Joó, P. Csiba and A. Bényei, J. Chem. Soc., Chem. Commun., 1993, 1602 RSC.
- A. Breslow, W. Maitra and D. Rideout, Tetrahedron Lett., 1983, 24, 1901 CrossRef.
- A. Meijer, S. Otto and J. B. F. N. Engberts, J. Org. Chem., 1998, 63, 8989 CrossRef CAS.
- O. Diebolt, C. Müller and D. Vogt, Catal. Sci. Technol., 2012, 2, 773 CAS.
- S. L. Desset, S. W. Reader and D. J. Cole-Hamilton, Green Chem., 2009, 11, 630 RSC.
- H. Nowothnick, A. Rost, T. Hameria, R. Schomäker, C. Müller and D. Vogt, Catal. Sci. Technol., 2013, 3, 600 CAS.
- Y. S. Varshavsky and T. G. Cherkasova, Zh. Neorg. Khim., 1967, 12, 1709 Search PubMed.
- The first synthesis of Rh/PAA was presented by Y. S. Varshvskii and T. G. Tcherkasova, on Ruscatal Conference, Abstracts, vol. 2, p. 123.
- D. Evans, G. Yagupsky and G. Wilkinson, J. Chem. Soc. A, 1968, 2660 RSC.
- (a) Y. S. Varshavsky, T. G. Cherkasova and I. S. Podkorytov, Inorg. Chem. Commun., 2004, 7, 489 Search PubMed; (b) Y. S. Varshavsky, T. G. Cherkasova, L. S. Podkorytov, A. A. Korlyukov, V. N. Khrustalev and A. B. Nikolskii, Russ. J. Coord. Chem., 2005, 31(2), 121 CrossRef.
- A. M. Trzeciak and J. J. Ziółkowski, J. Organomet. Chem., 1992, 429, 239 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |