A non-micellar synthesis of mesoporous carbon via spinodal decomposition†
Kimberly M. Nelsona,
Zhen-An Qiaob,
Shannon M. Mahurinb,
Richard T. Mayesb,
Craig A. Bridgesb and
Sheng Dai*ab
aDepartment of Chemistry, University of Tennessee, Knoxville, Tennessee 37996, USA
bChemical Sciences Division, Oak Ridge National Laboratory, Oak Ridge, Tennessee 37831, USA. E-mail: dais@orl.gov; Fax: +1-865-576-5235; Tel: +1-865-576-7307
First published on 6th May 2014
Abstract
Mesoporous carbons were prepared via spinodal decomposition of non-amphiphilic linear polyethylene glycol with phloroglucinol–formaldehyde resin under refluxing acidic ethanol conditions. By shifting the molecular weight and the concentration of polyethylene glycol, both mesopore size and volume can be tuned.
Traditional porous carbon materials are derived from coal, wood, biomass, or polymers.1 These carbons are typically microporous, and are formed from defects caused by heteroatoms eliminated during carbonization. Microporous carbons are often inadequate in terms of conductivity, mass transport, and structural integrity due to the remaining heteroatoms, restricted flow pathways, and lack of structural control. These deficiencies can be resolved by the introduction of mesoporosity, which makes them ideal for catalysis, batteries, supercapacitors, and adsorbents.2 Mesoporous carbons that can be tailored to optimize these applications are in high demand.
The standard templating synthesis utilizes methods that can be both costly and hazardous on the industrial scale.2a For instance, hard-templating of mesoporous carbons involves using a sacrificial silica template in combination with a carbon precursor, in which the template is etched after carbonization with harsh acids or bases (i.e. HF, NaOH) and a carbon inverse replica is revealed.3 Soft-templating synthesis tends to be less severe and is based on a self-assembly approach using block copolymer templating agents, which are removed via calcination.4 The block copolymers can be synthetically intensive to produce, which makes it very costly. While both of these methods produce well-defined mesopore size distributions and morphologies, they lack a facile route for mesopore development and a cost effective porogen that is relinquished by the process for industrial scale viability. Recently, Seo and Hillmyer demonstrated the polymerization induced microphase separation of trithiocarbonate terminated polylactide with vinylbenzene/divinylbenzene for mesoporous polymer synthesis via radical addition–fragmentation chain transfer.5 Polymerization quenched spinodal decomposition creates mesoscopic domains, and when combined with carbonization the pore forming polymer is effectively removed while the carbon precursor remains, preserving the mesostructure.
Herein, we establish a surfactant-free preparation of mesoporous carbon through the in situ polymerization of phloroglucinol–formaldehyde (PF) resins in the presence of polyethylene glycol (PEG) in acidic ethanol under reflux. The acid catalysed condensation polymerization of PF resins has previously served as a carbon precursor6 but the essence of our methodology resides in the synthesis of mesoporous carbon through spinodal decomposition instead of the traditional micellar self-assembly approaches.6a In lieu of triblock copolymers as templating agents, utilization of linear PEG provides a more cost effective alternative as a sacrificial templating agent.
In a typical run, phloroglucinol, formaldehyde, and PEG were mixed in ethanol under acidic conditions [see the ESI†]. Under refluxing conditions, PF–PEG aggregates were formed and precipitated. The PF–PEG solid was then dried and carbonized at 850 °C for 2 h under an Ar atmosphere at a rate of 2 °C min−1. Under these conditions, the near complete degradation of all the MWs of the PEG used can be achieved, and the carbonization of the remaining PF at this temperature could yield a material optimal for conductivity testing.7 The mesoporosity of the resulting carbon material was confirmed via scanning electron microscopy (SEM) and transmission electron microscopy (TEM) (Fig. 1 and S1–S8 in ESI†). The surface area of these materials was measured using nitrogen adsorption (Fig. 2).
On the basis of our results and literature reports, a possible mechanism for the formation of mesoporosity under the specified conditions is summarized in Scheme 1. Upon the addition of formaldehyde, acid catalyzed PF condensation polymerization occurs. As step-growth polymerization proceeds, the hydrophilic PF macromolecules undergo hydrogen-bonding interactions with the PEG polymers, leading to the formation of homogeneous PF–PEG aggregates, i.e. “polymer blend”. As the PF molecular weight increases, microphase separation of the aforementioned homogeneous aggregates into the mesoscopic domains via spinodal decomposition is evidenced by the co-continuous structure found in Fig. 1 but only microporosity in the PF sample without PEG addition (Fig. S10 in ESI†).8 The PF polymerization “chemically quenches” the reaction in the spinodal region as the phase composition changes and new phase miscibility conditions are established for the newly formed polymer–polymer blend.
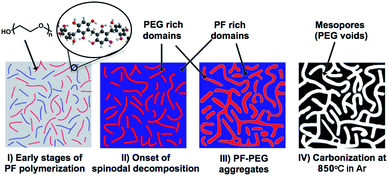 | ||
| Scheme 1 Schematic illustration of spinodal decomposition (I to III) and subsequent formation of mesoporous carbon (IV) from PF–PEG adduct. | ||
The acid is an essential component not only to the catalyzed polymerization of PF polymers but also to the interaction between the PF–PEG for driving the spinodal decomposition. The latter was evidenced by the formation of only microporous carbons from the samples prepared without the acid (Fig. S10 in ESI†). Acidic ethanol at increased temperature reduces polymer–polymer interactions, causing the end-to-end distance of the polymer chains to shrink. Eventually, cluster formation becomes favourable as the polymer chains collapse, leading to efficient spinodal decomposition.9 The as-synthesized material is non-porous (Fig. S10 in ESI†) after drying and curing. The subsequent carbonization at 850 °C under an inert Ar atmosphere destabilizes and decomposes the high oxygen containing PEG, revealing an inverse carbon replica. The mesoporosity is evident from the condensation step in the nitrogen adsorption isotherm with the desorption hysteresis characteristics of the type IV isotherm, shown in Fig. 2. Textural analysis was done using the Barrett, Joyner, and Halenda (BJH) method to calculate the pore size distributions according to the Kruk, Jaroniec, and Sayari (KJS) method, specific surface area was calculated using the Brunauer, Emmett, and Teller (BET) method, and micropore surface area and volume using the t-plot given in Tables 1 and 2.10 In comparison, carbon was produced at 850 °C using a similar procedure but with the triblock copolymer template. Pluronic F127 (MW 12.6k Da, PEO106PPO70PEO106), produced a similar micro- to mesopore ratio and pore volume but with an average pore size of 8.9 nm and a BET surface area of 518 m2 g−1.11 Although the pore size distribution for PF–PEG (14k Da) covers a much wider range of mesoporosity than that of Pluronic F127 templated PF resin, the adsorbed N2 contribution from micropores is only 0.066 cm3 g−1 compared to 0.12 cm3 g−1, attributing nearly 90% of the pore volume to mesopores instead of 81%.
| PEG MW [Da] | SBETa [m2 g−1] | Vtotal [cm3 g−1] | Vmesob [cm3 g−1] | Dmesoc [nm] |
|---|---|---|---|---|
| a Specific surface area calculated using the BET equation in the relative pressure range of 0.05–0.20.b The numbers in parentheses are percentages of mesopore volume out of the total pore volume.c Average pore diameter found at maximum differential pore volume. | ||||
| 2k | 360 | 0.197 | 0.098 (49.7) | 9 |
| 4k | 368 | 0.356 | 0.267 (75.0) | 14 |
| 8k | 372 | 0.480 | 0.395 (82.3) | 16 |
| 14k | 321 | 0.637 | 0.571 (89.6) | 25 |
| 20k | 368 | 0.374 | 0.285 (76.2) | 25 |
| 100k | 375 | 0.746 | 0.677 (91.2) | 31 |
| 200k | 375 | 0.629 | 0.558 (88.7) | 21 |
| PEG Conc.a [mM] | SBETb [m2 g−1] | Smesoc [m2 g−1] | VTotald [cm3 g−1] | Vmesoe [cm3 g−1] | Dmesof [nm] |
|---|---|---|---|---|---|
| a Phloroglucinol to 14k Da PEG weight ratio.b Specific surface area calculated using the BET equation in the relative pressure range of 0.05–0.20.c Mesopore surface area.d External surface area.e The numbers in parentheses are percentages of mesopore volume out of total pore volume.f Average pore diameter found at maximum differential pore volume. | |||||
| 2.9 | 392 | 83 | 0.244 | 0.121 (49.6) | 19 |
| 1.4 | 321 | 164 | 0.546 | 0.481 (88.1) | 25 |
| 0.63 | 321 | 159 | 0.637 | 0.571 (89.6) | 16 |
Under acidic ethanol reflux conditions, linear PEG chains agglomerate via spinodal decomposition in a similar fashion as the self-assembly of hydrophobic and hydrophilic blocks of Pluronic F127. In the Pluronic F127 templated carbon, the variation in mesopore size, pore size distribution discrepancies, and increased microporosity is due to the self-assembly of micelles during the reflux and curing process. The hydrogen bonding between the PF resin and PEO corona yields high microporosity in the resulting carbon; in contrast, PF resin has a much stronger interaction with the exterior of the PEG because of the separate polymer phases.
As seen in Table 1, mesoporosity extends to the carbon produced using this method with a molecular weight range of 2 to 200k Da PEG. Below 2k Da PEG, no mesoporosity was observed and microporosity was nominal (Fig. S10 in ESI†). At 2 kDa PEG, the desorption hysteresis closes at ∼0.45 P/Po, which is typically due to the cavitation in spherical pores. The small-angle X-ray scattering (SAXS) patterns of as-synthesized samples in Fig. S11† consist of one broad diffraction peak with q values of 0.092–0.17, and resolved features are not observed for higher reflections. This result suggests a worm-like mesopore dominated structure, which agrees well with the results observed from TEM images. When reviewing the pore size distribution from the carbon sample using the uppermost MW PEG, it is apparent that the broad pore size distribution is not a desirable characteristic for templated materials; although, this still provides a reasonably large effective range. The observed results in Fig. 2 confirmed that by shifting the molecular weight from low to high the average pore size increases. However, these values can not be reflected in the BJH average pore size calculation, as this method is used for mesopores in the range of 2 to 50 nm. With the pore size distributions for the larger molecular weight PEG, the calculation is not valid as it reaches this limit, considering the values fall well into the macropore domain.
The mesopore volume can then be adjusted through the concentration of PEG in solution. This approach allows the mesopore volume of the resulting carbon to be either raised or lowered, as shown in Fig. 3, for a specific application. Reducing the concentration from 2.9 mM to 1.4 mM PEG results in a minimal shift in pore size indicating that the concentration determines the amount of the corresponding polymer phase. In contrast, when the amount of PEG is further reduced to 0.63 mM, the microporosity of the sample is doubled and mesoporosity is reduced by nearly 40% (Table 2). By this reversal in porosity, decreasing the concentration of PEG shifts the composition ratio towards the binodal near a metastable region.12 Consequently, there is less defined spinodal decomposition occurring and fractal clusters are formed that generate the mesopores and micropores, respectively.13
In summary, using non surfactant linear PEG as a template for mesoporous carbon is reported. In contrast to prior soft templating approaches to mesoporous carbon, tailoring is limited only to the MW selection available. This material shows an improvement by reducing inherent microporosity while increasing pore size. By tuning the PF to PEG ratio, the mesopore volume can also be adjusted. These characteristics may be useful where mesoporosity is necessary for mass transport. Increased adsorption sites can be added using various means of activation to increase microporosity and add functionality.11,14 The ability to finely tune the mesoporosity of a carbon material through molecular weight and the concentration of PEG is relevant because of the novelty, particularly in comparison to traditional triblock copolymer templates where tuning would require more complex polymer synthesis. This concept is especially important for industrial scale synthesis.
Acknowledgements
This work was fully sponsored by the Division of Chemical Sciences, Geosciences, and Biosciences, Office of Basic Energy Sciences, U.S. Department of Energy.Notes and references
- (a) C. Liang, Z. Li and S. Dai, Angew. Chem., Int. Ed., 2008, 47, 3696–3717 CrossRef CAS PubMed; (b) R. L. Tseng and S. K. Tseng, J. Colloid Interface Sci., 2005, 287, 428–437 CrossRef CAS PubMed; (c) R. J. White, V. Budarin, R. Luque, J. H. Clark and D. J. Macquarrie, Chem. Soc. Rev., 2009, 38, 3401–3418 RSC.
- (a) Y. Shi, Y. Wan and D. Zhao, Chem. Soc. Rev., 2011, 40, 3854 RSC; (b) Y. P. Zhai, Y. Q. Dou, D. Y. Zhao, P. F. Fulvio, R. T. Mayes and S. Dai, Adv. Mater., 2011, 23, 4828–4850 CrossRef CAS PubMed.
- (a) A. H. Lu and F. Schüth, Adv. Mater., 2006, 18, 1793–1805 CrossRef CAS; (b) R. Ryoo, S. H. Joo and S. Jun, J. Phys. Chem. B, 1999, 103, 7743–7746 CrossRef CAS; (c) S. Jun, S. H. Joo, R. Ryoo, M. Kruk, M. Jaroniec, Z. Liu, T. Ohsuna and O. Terasaki, J. Am. Chem. Soc., 2000, 122, 10712–10713 CrossRef CAS.
- (a) C. Liang and S. Dai, J. Am. Chem. Soc., 2006, 128, 5316–5317 CrossRef CAS PubMed; (b) Y. Meng, D. Gu, F. Zhang, Y. Shi, L. Cheng, D. Feng, Z. Wu, Z. Chen, Y. Wan, A. Stein and D. Zhao, Chem. Mater., 2006, 18, 4447–4464 CrossRef CAS; (c) Y. Meng, D. Gu, F. Q. Zhang, Y. F. Shi, H. F. Yang, Z. Li, C. Z. Yu, B. Tu and D. Y. Zhao, Angew. Chem., Int. Ed., 2005, 44, 7053–7059 CrossRef CAS PubMed; (d) C. D. Liang, K. L. Hong, G. A. Guiochon, J. W. Mays and S. Dai, Angew. Chem., Int. Ed., 2004, 43, 5785–5789 CrossRef CAS PubMed.
- M. Seo and M. A. Hillmyer, Science, 2012, 336, 1422–1425 CrossRef CAS PubMed.
- (a) C. D. Liang and S. Dai, Chem. Mater., 2009, 21, 2115–2124 CrossRef CAS; (b) S. Tanaka, N. Nakatani, A. Doi and Y. Miyake, Carbon, 2011, 49, 3184–3189 CrossRef CAS PubMed; (c) T. Y. Ma, L. Liu and Z. Y. Yuan, Chem. Soc. Rev., 2013, 42, 3977–4003 RSC.
- (a) N. S. Vrandecic, M. Erceg, M. Jakic and I. Klaric, Thermochim. Acta, 2010, 498, 71–80 CrossRef CAS PubMed; (b) F. Y. Wang, C. C. M. Ma and W. J. Wu, J. Appl. Polym. Sci., 2001, 80, 188–196 CrossRef CAS.
- L. M. Robeson, Polymer blends: a comprehensive review, Hanser, Munich, Cincinnati, 2007 Search PubMed.
- (a) B. Hammouda, D. L. Ho and S. Kline, Macromolecules, 2004, 37, 6932–6937 CrossRef CAS; (b) P. Pang and P. Englezos, Colloids Surf., A, 2002, 204, 23–30 CrossRef CAS; (c) B. Briscoe, P. Luckham and S. Zhu, J. Appl. Polym. Sci., 1998, 70, 419–429 CrossRef CAS; (d) A. Mehrdad and R. Akbarzadeh, J. Chem. Eng. Data, 2010, 55, 2537–2541 CrossRef CAS.
- (a) M. Kruk and M. Jaroniec, Chem. Mater., 2001, 13, 3169–3183 CrossRef CAS; (b) M. Kruk, M. Jaroniec and A. Sayari, Langmuir, 1997, 13, 6267–6273 CrossRef CAS.
- X. Wang, J. S. Lee, C. Tsouris, D. W. DePaoli and S. Dai, J. Mater. Chem., 2010, 20, 4602–4607 RSC.
- T. Norisuye, M. Takeda and M. Shibayama, Macromolecules, 1998, 31, 5316–5322 CrossRef CAS.
- K. Binder, Colloid Polym. Sci., 1987, 265, 273–288 CAS.
- Z. Zhang, M. Xu, H. Wang and Z. Li, Chem. Eng. J., 2010, 160, 571–577 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details including synthesis of PEG templated phloroglucinol–formaldehyde derived carbon, associated SEM/TEM characterization, and SAXS analysis. See DOI: 10.1039/c4ra03558e |
| This journal is © The Royal Society of Chemistry 2014 |



