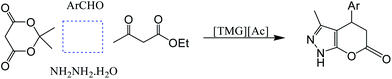Protic ionic liquid [TMG][Ac] as an efficient, homogeneous and recyclable catalyst for one-pot four-component synthesis of 2H-indazolo[2,1-b]phthalazine-triones and dihydro-1H-pyrano[2,3-c]pyrazol-6-ones
Hojat Veisi*a,
Abbas Amini Manesh*a,
Narges Khankhania and
Ramin Ghorbani-Vagheib
aDepartment of Chemistry, Payame Noor University (PNU), 19395-4697 Tehran, Iran. E-mail: hojatveisi@yahoo.com
bDepartment of Organic Chemistry, Faculty of Chemistry, Bu-Ali Sina University, Hamedan, Iran
First published on 20th May 2014
Abstract
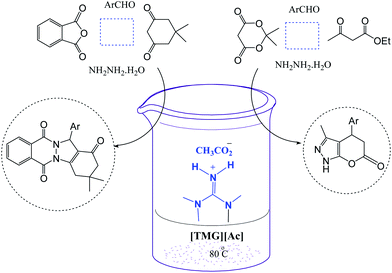
The mildly basic ionic liquid N,N,N,N-tetramethylguanidinium acetate [TMG][Ac] was found to be a very effective basic catalyst for the one-pot, four-component synthesis of 2H-indazolo[2,1-b]phthalazine-triones by condensation of phthalic anhydride, hydrazinium hydroxide, aromatic aldehydes, and dimedone in high yields at 80 °C. [TMG][Ac] catalyzed simple and efficient one-pot four-component reaction of Meldrums acid, ethyl acetoacetate, hydrazine hydrate, and aromatic aldehydes to give dihydro-1H-pyrano[2,3-c]pyrazol-6-ones is reported. The ionic liquid could be recycled several times without loss of efficiency with regards to the reaction times and yields.
Multi-component reactions (MCRs) have proven to be a valuable asset in organic and medicinal chemistry.1–7 Such protocols can be used for drug design, and drug discovery because of their simplicity, efficiency, and high selectivity.8,9 This environmentally friendly process can reduce the number of steps and synthesis of bioactive and complex molecules should be facile, fast, and efficient with minimal workup in this methodology.6,10
Currently, ionic liquids (ILs) have attracted great interest in the area of green chemistry due to their favorable properties such as negligible vapor pressure, wide liquid range, excellent salvation ability and feasible design ability.11 While considering ionic liquids as catalyst and their use in industrial processes, one major concern is cost. The cost of ionic liquid would be directly dependent on the price of the cations and anions that are used for their production.12 The research on new ILs bearing inert low coordinating and non-fluorinated anions represents a field of intense investigation in the chemistry of ILs.
Aza-containing heterocyclic compounds are widespread in nature, and their applications to pharmaceuticals, agrochemicals, and functional materials are becoming more and more important.13 Among a large variety of N-containing heterocyclic compounds, those containing hydrazine moiety as ‘fusion site’ have received considerable attention because of their pharmacological properties and clinical applications.14 Moreover, fused phthalazines and pyranopyrazole derivatives were found to possess multiple biological activities, such as antimicrobial,15 anticonvulsant,16 antifungal,17 anticancer,18 hypolipidemic,19 and anti-inflammatory activities.20 Nevertheless the development of new synthetic methods for the efficient preparation of heterocycles containing phthalazine and pyranopyrazole ring fragment is an interesting challenge. Recently, several elegant multicomponent strategies for the synthesis of 2H-indazolo[2,1-b]phthalazine-triones and pyranopyrazole-6-ones by multi component reactions utilizing catalysts have been reported.21–34
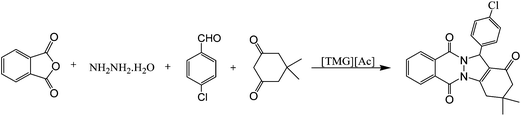
Due to our interest in the synthesis of heterocyclic compounds,35 herein we report a rapid and efficient one-pot four-component synthesis of some 2H-indazolo[2,1-b]phthalazine-triones and dihydro-1H-pyrano[2,3-c]pyrazol-6-ones in the presence of N,N,N,N-tetramethylguanidinium acetate [TMG][Ac] as mild basic ionic liquid catalyst (Scheme 1).
First, the four component reaction of hydrazine monohydrate, phthalic anhydride, dimedon, and 4-chlorobenzaldehyde in the presence of [TMG][Ac] was chosen as model. In order to establish the optimum conditions for this reaction, initially the influence of the reaction temperature, the amounts of catalyst and the reaction time were tested and optimized. For this purpose, a reaction between phthalic anhydride (1 mmol), hydrazinium hydroxide (1.1 mmol), dimedone (1 mmol) and 4-chlorobenzaldehyde (1 mmol) were examined (Table 1). To evaluate the effect of reaction temperature, system was carried out at different temperatures. At room temperature, the reaction rate was found to be very slow (Table 1, entries 1 and 15), and it was increased in higher temperatures. At 80 °C under solvent-free conditions, the reaction rate was found to be a maximum (Table 1, entry 9), and further increases in temperature did not show any enhancement (Table 1, entry 10). It should be noted that the optimal catalyst loading in the synthesis of 3,3-dimethyl-13-(4-chlorophenyl)-3,4-dihydro-1H-indazolo[1,2-b]phthalazine-1,6,11(2H,13H)-trione product peaked at a concentration of 10 mol%. When the amount of catalyst was lower, the yield of the product decreased (Table 1, entry 13), whereas raising the catalyst concentration did not lead to a pronounced increase to the product yield (Table 1, entry 12). In the absence of catalyst, no product was observed, even after prolonged reaction time (Table 1, entry 16). During our optimization studies, various solvents were examined and solvent-free conditions was found to be the most optimum in terms of reaction rate, and isolated yield, therefore optimized conditions is shown in Table 1.
| Entry | Solvent | Catalyst (mol%) | Temp. | Time (min) | Yield (%) |
|---|---|---|---|---|---|
| a Phthalic anhydride (1 mmol), hydrazinium hydroxide (1.1 mmol), dimedone (1 mmol) and 4-chlorobenzaldehyde (1 mmol). | |||||
| 1 | EtOH | 10 | 25 °C | 120 | 25 |
| 2 | EtOH | 10 | 80 °C | 120 | 65 |
| 3 | EtOH–H2O | 10 | 100 °C | 120 | 60 |
| 4 | DMF | 10 | 120 °C | 120 | 60 |
| 5 | CH2Cl2 | 10 | 70 °C | 120 | 40 |
| 6 | CH3CN | 10 | 90 °C | 90 | 55 |
| 7 | H2O | 10 | 100 °C | 120 | 60 |
| 8 | Toluene | 10 | 110 °C | 120 | 55 |
| 9 | Solvent-free | 10 | 80 °C | 15 | 96 |
| 10 | Solvent-free | 10 | 100 °C | 15 | 90 |
| 11 | Solvent-free | 10 | 60 °C | 15 | 70 |
| 12 | Solvent-free | 20 | 80 °C | 15 | 96 |
| 13 | Solvent-free | 5 | 80 °C | 15 | 70 |
| 14 | Solvent-free | 5 | 80 °C | 120 | 75 |
| 15 | Solvent-free | 10 | 25 °C | 120 | Trace |
| 16 | Solvent-free | 0 | 80 °C | 120 | Trace |
With a reliable set of conditions in hand (Table 1, entry 9), the scope and generality of the developed protocol with respect to various aldehydes was investigated in the presence of 10 mol% of ionic liquid at 80 °C under solvent-free conditions. The results are summarized in Table 2.
| Entry | Ar/R | Time (min) | Yielda (%) | Mp (°C) |
|---|---|---|---|---|
| a Yield refers to isolated product. | ||||
| 1 | C6H5 | 15 | 92 | 202–204 (ref. 33) |
| 2 | 4-Me-C6H4 | 20 | 96 | 226–228 (ref. 33) |
| 3 | 4-MeO-C6H4 | 15 | 90 | 217–219 (ref. 27) |
| 4 | 4-Cl-C6H4 | 15 | 96 | 263–265 (ref. 33) |
| 5 | 2,4-Cl2-C6H3 | 10 | 96 | 218–220 (ref. 33) |
| 6 | 4-NO2-C6H4 | 12 | 95 | 224–226 (ref. 27) |
| 7 | 3-NO2-C6H4 | 10 | 98 | 269–271 (ref. 27) |
| 8 | 4-Br-C6H4 | 12 | 93 | 280–282 (ref. 33) |
| 9 | 2-Naphthyl | 15 | 90 | 250–252 (ref. 27) |
| 10 | Propanal | 30 | 65 | 146–148 (ref. 27) |
| 11 | Butanal | 30 | 60 | 136–138 (ref. 27) |
| 12 | Heptanal | 30 | 55 | 80–82 (ref. 27) |
As shown in Table 2, a series of aliphatic and aromatic aldehydes containing either electron-withdrawing or electron-donating substituents successfully reacted and afforded good to high yields of products with high purity, at 80 °C under solvent-free conditions. The nature and electronic properties of the aldehyde substrates affect the conversion rate and yield. Aromatic aldehydes (Table 2, entries 1–9) react faster and in higher yield than the aliphatic aldehydes (Table 2, entries 10–12).
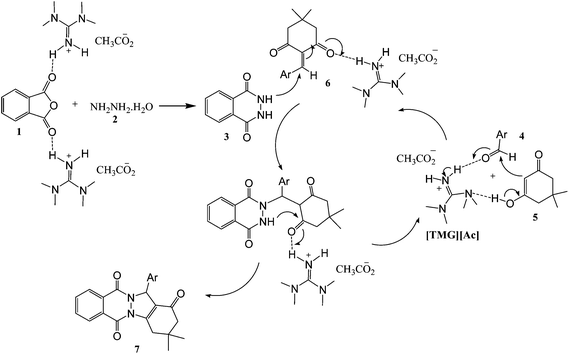
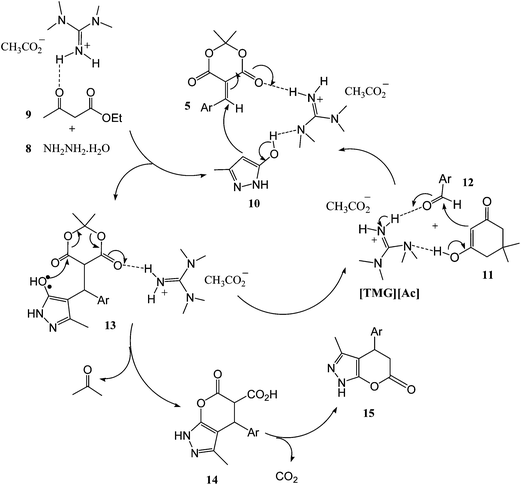
A possible mechanism for the formation of 2H-indazolo[2,1-b]phthalazine-triones in the presence of [TMG][Ac] is proposed in Scheme 2. The reaction occurs via initial formation of the phthalhydrazide 3 by nucleophilic addition of hydrazinium hydroxide 2 to phthalic anhydride 1 followed by dehydration. The second step involves initial formation of heterodiene 6 by standard Knoevenagel condensation of dimedone 5 and aldehyde 4. Subsequent Michael-type addition of the phthalhydrazide 3–6 followed by cyclization affords the corresponding product 7 (Scheme 2).
The ionic liquid, [TMG][Ac], could be recovered from the aqueous extracts of the reaction mixtures by evaporation of water under reduced pressure. It retains almost all the initial activity after recovery, when reused in the next successive cycles. For instance running the model reaction of phthalic anhydride, hydrazinium hydroxide, dimedone and 4-chlorobenzaldehyde in 10 mol% of fresh, first recycled, and second recycled [TMG][Ac] at 80 °C under solvent-free conditions has given, respectively, 96%, 94%, and 90% yield of the product.
In continuation of work, we also report a rapid and efficient one-pot four-component synthesis of some 3-methyl-4-aryl-4,5-dihydro-1H-pyrano[2,3-c]pyrazol-6-ones by the reaction of aromatic aldehydes, Meldrums acid, hydrazine hydrate, and ethyl acetoacetate in the presence of N,N,N,N-tetramethylguanidinium acetate [TMG][Ac] as green and mild basic ionic liquid medium (Scheme 1).
To find the best conditions, we carried out the reaction between 3,4,5-trimethoxybenzaldehyde, ethyl acetoacetate, Meldrums acid, and hydrazine hydrate in the presence of 10 mol% of [TMG][Ac] at 80 °C under solvent-free conditions. We found that, the product was obtained in very high yield (98%) within 0.5 h (Table 3, entry 6). After optimization of the conditions for the model reaction, a range of different aldehydes were screened to explore the scope of this protocol. As shown in Table 3, the reaction is compatible with an array of arylaldehydes bearing either electron-withdrawing or electron-donating substituents.
| Entry | Ar/R | Time (h) | Yielda (%) | Mp (°C) |
|---|---|---|---|---|
| a Yield refers to isolated product. | ||||
| 1 | 4-MeO-C6H4 | 0.5 | 96 | 158–160 (ref. 30) |
| 2 | 4-F-C6H4 | 1 | 92 | 180–182 (ref. 30) |
| 3 | 2-Cl-C6H4 | 1 | 90 | 142–144 (ref. 30) |
| 4 | 2-OH-C6H4 | 1 | 95 | 234–236 (ref. 30) |
| 5 | 3-NO2-C6H4 | 1.2 | 95 | 212–214 (ref. 30) |
| 6 | 3,4,5-(MeO)3-C6H2 | 0.5 | 98 | 204–206 (ref. 30) |
| 7 | Butanal | 24 | 0 | — |
A possible pathway is proposed in Scheme 3. The synthesis is likely initiated by [TMG][Ac], which upon removing a proton from Meldrum's acid 11 promotes a Knoevenagel condensation with the aldehyde 12, resulting in formation of the intermediate 13. This intermediate subsequently undergoes a Michael type addition with pyrazolone 10 (the pyrazolone 10 is produced by the reaction between 8 and 9) to produce the adduct 13. Cyclization of 13 via a translactonization reaction leads to liberation of an acetone molecule leaving the initial product 14 bearing a carboxyl group at the 3-position. Spontaneous decarboxylation of the product 14 affords the corresponding product 15.
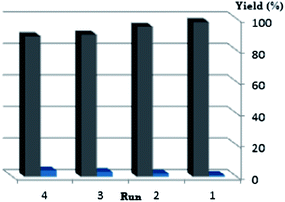
We also investigated the recycling of the ionic liquid under solvent-free conditions using a model reaction of hydrazine monohydrate, 3,4,5-trimethoxybenzaldehyde, ethyl acetoacetate and Meldrums acid for the preparation of 3-methyl-4-(3′,4′,5′-trimethoxyphenyl)-4,5-dihydro-1H-pyrano[2,3-c]pyrazol-6-one in the presence of [TMG][Ac] at 80 °C. After completion of the reaction, water was added and the precipitated mixture was filtered off for separation of crude products. After washing the solid products with water completely, the water containing ionic liquid (IL is soluble in water) was evaporated under reduced pressure and ionic liquid was recovered and reused (Fig. 1). The recovered catalysts were reused four runs without any loss of their activities.
In summary, we have demonstrated a simple, efficient, and a one-pot four-component protocol for the synthesis of 2H-indazolo[2,1-b]phthalazine-triones and dihydro-1H-pyrano[2,3-c]pyrazol-6-ones in the presence of N,N,N,N-tetramethylguanidinium acetate [TMG][Ac] as mild basic ionic liquid catalyst at 80 °C. The attractive features of this protocol are simple procedure, cleaner reaction, use of inexpensive and reusable ionic liquid as catalyst. Satisfactory yields of products, as well as a simple reactions, isolation, and purification of the products make it a useful protocol for the synthesis of these classes of compounds.
Preparation of [TMG][Ac]
Protic ILs were synthesized simply by neutralization of N,N,N,N-tetramethylguanidin with corresponding acetic acid. In a typical reaction, 0.1 mol N,N,N,N-tetramethylguanidin dissolved in 10 ml methanol was put into a 100 ml flask, and 0.1 mol acetic acid was slowly added under stirring. The solution was stirred at room temperature for 24 h to complete the reaction, and then the methanol was removed at 50 °C under reduced pressure to give a colorless viscous liquid.Typical procedure for the synthesis of 2H-indazolo[2,1-b]phthalazine-trione derivatives
Hydrazinium hydroxide (1.1 mmol), phthalic anhydride (1 mmol), dimedone (1 mmol), aldehyde (1 mmol) and [TMG][Ac] (10 mol%) was heated at 80 °C. The completion of reaction is monitored on TLC. The 2H-indazolo[2,1-b]phthalazine-1,6,11(13H)-trione derivatives were synthesized. After satisfactory completion of the reaction, the mixture was cooled to room temperature and H2O (10 ml) was added to this mixture and was shaken for a few minutes to dissolve [TMG][Ac]. The crude product (insoluble in water) was filtered and recrystallized from hot ethanol for more purification. The desired pure products were characterized by comparison of their physical data with those of known compounds. In order to recover the catalyst, H2O was evaporated under reduced pressure, and the resulting ionic liquid was washed with di-ethyl ether, and dried. The recovered catalyst was reused 5 times.Typical procedure for the synthesis of dihydro-1H-pyrano[2,3-c]pyrazol-6-ones derivatives
In a 50 ml round bottom flask, a mixture of hydrazine hydrate (1.2 mmol), ethyl acetoacetate (1 mmol), and [TMG][Ac] (10 mol%) were taken, the mixture was stirred in an oil bath and heated for 15 min at 80 °C; aromatic aldehyde (1 mmol) and Meldrums acid (1 mmol) were then added and heating was continued for the remaining time (Table 3). After completion of the reaction, the mixture was cooled to room temperature and H2O (10 ml) was added to this mixture and was shaken for a few minutes to dissolve [TMG][Ac]. The crude product (insoluble in water) was filtered and recrystallized from hot ethanol 80% for more purification. In order to recover the catalyst, H2O was evaporated under reduced pressure, and the resulting ionic liquid was washed with di-ethyl ether, and dried. The recovered catalyst was reused next times.Analytical data for selected products
3,3-Dimethyl-13-(3-nitrophenyl)-3,4-dihydro-2H-indazolo [1,2-b]phthalazine-1,6,11(13H)-trione (Table 2, entry 7)
Yellow powder (98%); mp 269–271 °C; IR (KBr): νmax (cm−1) 2975, 1682, 1660, 1625; 1H NMR (300 MHz, CDCl3): δ 1.24 (6H, s, 2Me), 2.36 (2H, s, CH2C), 3.24–3.47 (2H, CH2CO), 6.53 (1H, s, CHN), 7.53–8.4 (8H, m, Ph); 13C NMR (300 MHz, CDCl3): δ 28.4, 28.7, 34.7, 38.0, 50.8, 64.1, 117.1, 121.5, 123.7, 127.7, 128.2, 128.6, 128.9, 129.6, 133.9, 134.2, 134.7, 138.4, 148.5, 150.8, 154.3, 155.9, 192.1.(3′,4′,5′-Trimethoxyphenyl)-4,5-dihydro-1H-pyrano[2,3-c]pyrazol-6-one (Table 3, entry 6)
Yellow solid (98%); mp 204–206 °C: IR (KBr): νmax (cm−1) 3372, 2972, 1735, 1691, 1620, 1569, 1498, 1448, 1375, 1235, 1173; 1H NMR (400 MHz, DMSO-d6): δ 2.87 (d, 2H, CH2), 3.42 (s, 3H, Me), 3.59 (s, 3H, OCH3), 3.62 (s, 3H, OCH3), 3.82 (s, 3H, OCH3), 4.81 (t, 1H, CH), 6.61 (s, 2H, Ph), 10.84 (s, 1H, NH); 13C NMR (100 MHz, DMSO-d6): δ 169.8, 154.6, 147.3, 132.8, 128.8, 114.8, 108.1, 145.0, 136.8, 117.8, 60.1, 56.5, 54.7, 29.7, 14.7.Acknowledgements
We are thankful to Payame Noor University (PNU) for partial support of this work.References
- J. Zhu and H. Bienayme, Multicomponent Reactions, Wiley-VCH, Weinheim, Germany, 2005 Search PubMed.
- A. Dömling, Chem. Rev., 2006, 106, 17–89 CrossRef PubMed.
- S. Jimenez-Alonso, H. Chavez, A. Estevez-Braan, A. Ravelo, G. Feresin and A. Tapia, Tetrahedron, 2008, 64, 8938 CrossRef PubMed.
- D. F. Tejedor and G. Tellado, Chem. Soc. Rev., 2007, 36, 484 RSC.
- R. V. A. Orru and M. de Greef, Synthesis, 2003, 10, 1471 CrossRef PubMed.
- L. Weber, Drug Discovery Today, 2002, 7, 143 CrossRef.
- A. Domling and I. Ugi, Angew. Chem., Int. Ed., 2000, 39, 3169 CrossRef.
- A. Nefzi, J. M. Ostresh and R. A. Houghten, Chem. Rev., 1997, 97, 449 CrossRef PubMed.
- L. A. Thompson, Curr. Opin. Chem. Biol., 2000, 4, 324 CrossRef.
- A. Domling, Curr. Opin. Chem. Biol., 2000, 6, 306 CrossRef.
- J. Dupont, R. F. de Souza and P. A. Z. Auarez, Chem. Rev., 2002, 102, 3667 CrossRef PubMed.
- P. Wasserscheid and T. Welton, Ionic Liquid in Synthesis, Wiley-VCH, 2004 Search PubMed.
- V. P. Litvinov, Russ. Chem. Rev., 2003, 72, 69 CrossRef PubMed.
- R. A. Clement, J. Org. Chem., 1960, 25, 1724 CrossRef.
- (a) S. S. El-Saka, A. H. Soliman and A. M. Imam, Afinidad, 2009, 66, 167 Search PubMed; (b) K. Kuppusamy and P. Kasi, Tetrahedron Lett., 2010, 51, 3312 CrossRef PubMed; (c) E. S. El-Tamany, F. A. El-Shahed and B. H. Mohamed, J. Serb. Chem. Soc., 1999, 64, 9 Search PubMed; (d) J. L. Wang, D. Liu, Z. J. Zhang, S. Shan, X. Han, S. M. Srini vasula, C. M. Croce, E. S. Alnemri and Z. Huang, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 7124 CrossRef; (e) F. M. Abdelrazek, P. Metz, N. H. Metwally and S. F. El-Mahrouky, Arch. Pharm., 2006, 339, 456 CrossRef PubMed.
- L. Zhang, L. P. Guan, X. Y. Sun, C. X. Wei, K. Y. Chai and Z. S. Quan, Chem. Biol. Drug Des., 2009, 73, 313 Search PubMed.
- C. K. Ryu, R. E. Park, M. Y. Ma and J. H. Nho, Bioorg. Med. Chem. Lett., 2007, 17, 2577 CrossRef PubMed.
- J. Li, Y. F. Zhao, X. Y. Yuan, J. X. Xu and P. Gong, Molecules, 2006, 11, 574 CrossRef.
- R. A. Izydore and I. H. Hall, U.S. Patent 4,866,058, file date, 12 September 1989, Chem. Abstr, 1990, 112, 151876X.
- J. Sinkkonen, V. Ovcharenko, K. N. Zelenin, I. P. Bezhan, B. A. Chakchir, F. Al-Assar and K. Pihlaja, Eur. J. Org. Chem., 2002, 2046 CrossRef.
- M. Sayyafi, M. Seyyedhamzeh, H. R. Khavasi and A. Bazgir, Tetrahedron, 2008, 64, 2375 CrossRef PubMed.
- J. M. Khurana and M. Devanshi, Tetrahedron Lett., 2009, 50, 7300 CrossRef PubMed.
- H. R. Shaterian, M. Ghashang and M. Feyzi, Appl. Catal., A, 2008, 345, 128 CrossRef PubMed.
- E. Mosaddegh and A. Hassankhani, Tetrahedron Lett., 2011, 52, 488 CrossRef PubMed.
- R. Fazaeli, H. Aliyan and N. Fazaeli, Open Catal. J., 2010, 3, 14 CrossRef.
- G. Sabitha, C. Srinivas, A. Raghavendar and J. S. Yadav, Helv. Chim. Acta, 2010, 93, 1375 CrossRef.
- R. Ghorbani-Vaghei, R. Karimi-Nami, Z. Toghraei-Semiromi, M. Amiri and M. Ghavidel, Tetrahedron, 2011, 67, 1930 CrossRef PubMed.
- S. Gaurav, K. V. Rajiv, K. V. Girijesh and M. Shankar Singh, Tetrahedron Lett., 2011, 52, 7195 CrossRef PubMed.
- W. Xiao, M. Wei-Wei, W. Li-Qiang and Y. Fu-Lin, J. Chin. Chem. Soc., 2010, 57, 1341 Search PubMed.
- S. H. S. Azzam and M. A. Pasha, Tetrahedron Lett., 2012, 53, 6834 CrossRef PubMed.
- M. V. Reddy, G. C. S. Reddy and Y. T. Jeong, Tetrahedron, 2012, 68, 6820 CrossRef PubMed.
- A. Hasaninejad, M. Rasekhi Kazerooni and A. Zare, Catal. Today, 2012, 196, 148 CrossRef PubMed.
- M. Kidwai, A. Jahan, R. Chauhan and N. K. Mishra, Tetrahedron Lett., 2012, 53, 1728 CrossRef PubMed.
- (a) Y. A. Sharanin, L. G. Sharanina and V. V. Puzanova, Zh. Org. Khim., 1983, 19, 2609 Search PubMed; (b) L. A. Rodinovskaya, A. V. Gromova, A. M. Shestopalov and V. N. Nesterov, Russ. Chem. Bull., Int. Ed., 2003, 52, 2207 CrossRef; (c) H. Junek and H. Aigner, Chem. Ber., 1973, 106, 914 CrossRef.
- (a) H. Veisi and R. Ghorbani-Vaghei, Tetrahedron, 2010, 66, 7445 CrossRef PubMed; (b) R. Ghorbani-Vaghei and H. Veisi, Synthesis, 2009, 945 CrossRef PubMed; (c) H. Veisi, Synthesis, 2010, 2631 CrossRef PubMed; (d) H. Veisi, S. Hemmati and H. Veisi, J. Chin. Chem. Soc., 2009, 56, 240 Search PubMed.
| This journal is © The Royal Society of Chemistry 2014 |