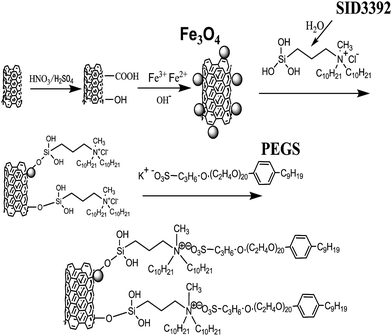
A functional liquid-like multiwalled carbon nanotube derivative in the absence of solvent and its application in nanocomposites
Yaping Zheng*,
Ruilu Yang,
Fei Wu,
Dewang Li,
Nan Wang and
Aibo Zhang
School of Natural and Applied Science, Northwestern Polytechnical University, Xi'an, Shaanxi 710072, China. E-mail: zhengyp@nwpu.edu.cn; Fax: +86-29-88431688
First published on 9th June 2014
Abstract
A multiwall carbon nanotube (MWCNT) derivative with liquid-like behaviour at room temperature was prepared by attaching Fe3O4 on the surface of MWCNT and employing tertiary amine terminated organosilanes as a corona and sulfonic-acid as a canopy. The MWCNT derivative is a Newtonian fluid at low shear rate. It has relative low viscosity at room temperature (3.87 Pa s at 25.7 °C). It shows good solubility in organic solvents such as ethanol, acetone and dichloroethane. Transmission electron microscopy (TEM) images confirmed the good dispersion of the MWCNT derivative in the solvent and the epoxy matrix. The MWCNT derivative is a super-paramagnetic material with a specific magnetization of 2.68 emu g−1. According to the TEM image, the derivative can be aligned in the epoxy matrix under a magnetic field. The MWCNT derivative can improve the impact toughness of pure epoxy by 194% and the glass transition temperature (Tg) by 16.9 °C. The MWCNT derivative has many novel properties and can be used in nanocomposites.
Introduction
One of the major challenges for the application of multiwall carbon nanotube (MWCNT) is to disperse MWCNTs uniformly in solvent and polymer matrix.1–7 Therefore, a versatile way was developed to keep nanoparticles isolated and uniformly dispersed in solvent and polymer matrix.8 By grafting a short oligomer onto the surface of the MWCNT, we can obtain a liquid-like MWCNT derivative in the absence of solvent, which can be dispersed well in solvent and polymer matrix.9,10Several studies have been reported in the field of liquid-like MWCNT derivatives. Chuanxi Xiong prepared PEG-functionalized CNTs (PEG-CNTs) with higher functional density and a smaller aspect ratio.11 They found that the novel controllable rheology liquid-like MWCNT derivative could be achieved by controlling the oxidation time of carbon nanotubes. Notably, they also prepared 500 nm-long functional carbon nanotubes, which displayed liquid-like behaviour in the solvent-free system.12 Robert prepared a MWCNT derivative by a radical-based reaction.13 In addition, a liquid-like MWCNT derivative could also be obtained through hydrogen bonds and the steric effect of a pluronic copolymer.14 The material was in waxy solid state at room temperature, which melted and behaved like a liquid at 45 °C. There are also many liquid-like derivatives, which are based on different materials like fullerenes and ZnO as a core.15–18 Rodriguez19 reported a SiO2 nanoparticle derivative based on 18 nm diameter silica nanoparticles as the core, sulfonic acid-terminated corona and tertiary amines as the canopy. The viscosity of the fluid can be controlled by varying the volume fraction of the core, the canopy geometry and molecular weight. Michinobu20 prepared a solvent-free room temperature liquid-like fullerenes by attaching a single substituent of a 1,3,5-tris(alkyloxy)benzene unit to C60 or C70 under the Prato conditions. By attaching branched low-viscosity aliphatic chains to the anthracene core, Sukumaran Santhosh Babu21 reported nonvolatile, blue-emitting and highly stable liquid-like anthracenes at room temperature, in which acceptor dyes can be doped to tune the luminescence color. However, a liquid-like MWCNT/Fe3O4 derivative has not been attempted before. In this paper, we prepared a liquid-like derivative by attaching organosilane SID3392 and sulfonic-acid PEGS onto the surface of MWCNT//Fe3O4 (prepared by chemical co-precipitation).
Many scientists have explored the application of MWCNT in polymeric nanocomposites.22,23 There are two common ways to disperse solid MWCNT uniformly in the polymer matrix. The first one is to use a solution processing technique. MWCNTs were dispersed in ethanol with an epoxy monomer and a curing agent by sonication and mechanically. After evaporation of the volatile solvent, the mixture was poured into molds and cured.24 The second method is melt mixing. Jin25 blended MWCNTs and PMMA in a laboratory mixing molder at a speed of 120 rpm (200 °C). The mixed samples were then compressed under pressure at 210 °C using a hydraulic press to yield composite films. However, both techniques have shortcomings. The melt compounding processing often yields a poor dispersion of MWCNTs since the viscosity of the MWCNT and the polymer mixture is very high.26 The solution processing technique, with its expensive organic solvent and complex post-treatment, induces defects and hampers the mechanical property of composites.27
Therefore, a new way to prepare a MWCNT/polymer nanocomposite was developed. Li28 used a liquid-like MWCNT derivative to prepare liquid-like MWCNT/PA11 nanocomposites. The fracture elongation of the liquid-like MWCNT/PA11 nanocomposites maintained the range of 140–260%. The tensile modulus of the nanocomposite had increased by about 22% when the loading of liquid-like MWCNT was 0.8 wt%. Yang29 reported that the liquid-like MWCNT derivative improved the tensile property of the epoxy. When liquid-like MWCNT derivative content was 0.5 wt%, the Young's modulus, tensile strength, failure strain and toughness of epoxy nanocomposites were increased by 28.4%, 22.9%, 24.1% and 66.1%, respectively. However, none of these papers ever discussed heat resistance of the nanocomposites and the effect of the MWCNT derivative on the curing process of the epoxy.
Several studies have been reported in the field of alignment of MWCNT in a polymer. Camponeschi's research showed that pristine MWCNT aligned in the epoxy matrix under a magnetic field of 17 T, the properties of the resultant composites are superior to those that were not exposed to a magnetic field.30 Iron oxide decorated CNT can also be aligned under a magnetic field of 0.4 T.31 According to Abdalla's research,32 pristine MWCNTs were partially aligned in epoxy under a magnetic field of 9.4 T. The modulus parallel to the alignment direction, as measured by dynamic mechanical analysis showed significant anisotropy with 72% increase over the neat resin and 24% increase perpendicular to the alignment direction. Sharma33 aligned SWCNT and MWCNT in the PC matrix by applying an external magnetic field of 1200 G. Magnetically aligned CNT/PC nanocomposites had better gas permeability than the randomly oriented CNT/PC nanocomposites. H2 was found to be more selective than N2 and CO2. Although there are many works reported about alignment of solid MWCNT in the polymer matrix, alignment of a liquid-like MWCNT derivative has not been attempted before.
In this paper, we have prepared a liquid-like MWCNT derivative utilizing MWCNT/Fe3O4 as a core. The liquid-like MWCNT/Fe3O4 derivative was aligned in the epoxy matrix to prepare nanocomposites. The toughness, glass transition temperature and curing process were studied. The findings reported here can provide fundamental data about the structure control of the MWCNT-based derivative, and their output properties determined by their structure can also be tuned according to the structure-property relationship.
Experimental section
Materials and preparation
The MWCNTs (diameter 20–30 nm, length 5–15 μm) used in this study were synthesized by chemical vapor deposition (CVD) and provided by Chengdu organic chemicals Co., Ltd. FeCl3·6H2O (wt% > 99.0%) and FeCl2·4H2O (wt% > 99.7%) were obtained from Tianjing Organics. N,N-Didecyl-N-methyl-N-(3-trimethoxysilylpropyl) ammonium chloride (SID3392) was from Gelest. Inc. Poly(ethylene glycol)4-nonylphenyl-3-sulfopropyl ether and potassium salt (PEGS) were purchased from Aldrich. Methyl tetrahydrophthalic anhydride (METHPA) was from GuangZhou Weibo Chemistry Co. Ltd. 2,4,6-Tri(dimethylaminomethyl) phenol (DMP-30) was purchased from Aladdin Chemistry Co. Ltd. The epoxy resin (CYD-128) and curing agent 593 were from Yueyang Petrochemical Co. Ltd. Other analytical grade chemicals were H2SO4, HNO3, NH3·H2O, ethanol, methanol, acetone, methylbenzene, tetrahydrofuran, dichloroethane and ether.To begin with, MWCNTs were modified with H2SO4–HNO3 (volume ratio 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for three hours at 70 °C. The products were washed with the deionized water in order to remove residual acid and dried at 70 °C for 24 h. FeCl2·4H2O and FeCl3·6H2O with a molecule ratio of 1
1) for three hours at 70 °C. The products were washed with the deionized water in order to remove residual acid and dried at 70 °C for 24 h. FeCl2·4H2O and FeCl3·6H2O with a molecule ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.75 were dissolved completely in de-ionized water. Subsequently, acid MWCNTs were added to the suspension, stirred, and sonicated for 2 h at 30 °C. Ammonia (5%) was added slowly into the solution and reacted for 30 min. MWCNT/Fe3O4 hybrids were deposited at the bottom of the beaker under a magnetic field. MWCNT/Fe3O4 hybrids were rinsed with de-ionized water until pH = 7, dried at 60 °C, and grinded to powder.
1.75 were dissolved completely in de-ionized water. Subsequently, acid MWCNTs were added to the suspension, stirred, and sonicated for 2 h at 30 °C. Ammonia (5%) was added slowly into the solution and reacted for 30 min. MWCNT/Fe3O4 hybrids were deposited at the bottom of the beaker under a magnetic field. MWCNT/Fe3O4 hybrids were rinsed with de-ionized water until pH = 7, dried at 60 °C, and grinded to powder.
Then MWCNT/Fe3O4 hybrids (0.5 g) were mixed with 20 mL deionized water through ultrasound dispersion for 30 min. Ammonia (5%) was added into the mixture until the pH was 10. After that, SID3392 methanol solution (3 mL, weight fraction 40–42%) was added into the mixture dropwise. The black precipitate, which was formed immediately, was aged for 24 h at room temperature by shaking it intermittently. Then, the solvents were discarded and the solid was rinsed three times with water and ethanol. The solid was dispersed in tetramethylene oxide and upper layer solution was collected and dried at 50 °C.
At last, the product (1.2 g) was dispersed in 100 mL deionized water and PEGS ethanol solution (15 g, weight fraction 20%) was added into the solution. The reaction proceeded under mechanical stirring at 40 °C for 24 h, and the product was dried at 50 °C. The product was dissolved in acetone and centrifuged at 1000 rpm for 10 min. Solids on the surface of the tube were discarded. Finally, the supernatant liquid was collected, concentrated and dried at 50 °C.
Then, liquid-like derivative was aligned in the epoxy matrix, which was dispersed in the epoxy resin with sonication for 30 min at 60 °C. The curing agent 593 was added into the mixture and stirred slowly. The mixture were placed under vacuum at 36 °C to get rid of the bubbles. After that, the mixture was immediately poured into a mold, and a 3 T magnetic field was applied for 24 h. The liquid-like MWCNT derivative was added into the epoxy resin at 65 °C and sonicated for 30 min. The mixture was cured in the vacuum oven with METHPA as a curing agent and DMP-30 as an accelerating agent. The system was cured at 90 °C for 90 min, followed by 100 °C for 30 min, 110 °C for 30 min, 120 °C for 30 min and 140 °C for 90 min.
Finally, the liquid-like MWCNT derivative/epoxy nanocomposites were prepared, and the impact toughness and Tg of the liquid-like MWCNT derivative/epoxy nanocomposites were tested.
Characterization
The surface groups on the MWCNT derivative were investigated by Fourier transform infrared spectrometer (FTIR) (Mode: WQF-310) using KBr pellets. The product was characterized by X-ray diffraction (XRD, Rigaku, model D/max-2500 system). Transmission electron microscope (TEM) images of MWCNT, MWCNT/Fe3O4 and MWCNT derivative were obtained at an accelerating voltage of 100 kV using the JEM-2100 instrument after a few drops of sample/ethanol solution were placed on a copper grid and dried. X-ray photoelectron spectroscopy (XPS) was performed with the Kratos Axis Ultra DLD instrument after a few MWCNT/Fe3O4 nanoparticles were placed on a conductive blanket. The wide scan ranged from 0 eV to 760 eV. Differential scanning calorimetry (DSC) traces of the MWCNT derivative and nanocomposite were collected using a Q1000 TA instrument at a heating rate of 10 °C min−1. Thermogravimetric analysis (TGA) measurements were performed under N2 flow by using a TGAQ50 TA instrument. Rheological properties were studied by the rheometer of TA instrument (AR-2000). The cone-plate geometry with a cone diameter of 40 mm and a cone angle of 2° was used. A steady flow test was carried out at a temperature of 25 °C. Then, a temperature ramp test was carried out at 100 s−1. After that, frequency sweep test was carried out at 20 °C and constant strain of 5%. Finally, the temperature sweep test was carried out at the frequency of 50 s−1 and constant strain of 5%. Magnetic study was performed by a vibrating sample magnetometer (VSM, Riken Denshi, BHV-525). Impact toughness of the nanocomposites with different weight fraction of the MWCNT derivative was tested on the universal testing machine (CMT-5105), and the test followed standard GB/T 2567-2008.Results and discussion
Characterization of the liquid-like MWCNT derivative
The liquid-like MWCNT derivative was synthesized through four reaction steps (Fig. 1). The product exhibits liquid-like behaviour in the absence of solvent (Fig. 2).The groups on the surface of these hybrids were studied by FTIR spectra (Fig. 3). For the FTIR spectra of carboxylic MWCNT (Fig. 3(b)), the absorption peak at 3310 cm−1 is for hydrogen bonding of hydroxyl (–OH). The one at 1654 cm−1 is related to the formation of hydrogen bonding between the carbonyl of carboxyl and the hydroxyl of another carboxyl. The absorption peak of the C–O bond is observed at 1392 cm−1. These absorption peaks indicate that the carboxyl groups (–COOH) and hydroxyl groups (–OH) have been successfully decorated onto the surface of the MWCNT. For the FTIR curve of MWCNT/Fe3O4 (Fig. 3(c)), the absorption peak of Fe–O bond vibration at 570 cm−1 confirmed that Fe3O4 has been attached onto the surface of MWCNT. As for the liquid-like MWCNT derivative (Fig. 3(d)), there are absorption peaks of Si–OH at 950 cm−1 (ref. 34) and absorption peaks of Si–C at 840 cm−1.35 These absorption peaks indicate that SID3392 has been attached onto the surface of MWCNT/Fe3O4. Moreover, the absorption peak at 1112 cm−1 is assigned to the asymmetric stretching vibration of –CH2–O–CH2, suggesting that PEGS has also been decorated. The peak at 1728 cm−1 was assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations of the –COOH groups. Several absorption bands in the region of 1500–1200 cm−1 were assigned to the O–H deformations of the C–OH groups and stretching vibrations. The absorption peaks at 720 and 1969 cm−1 represent SO3− and R3NH+, respectively.36 Because the intensity of these polar groups is relatively strong, the Fe–O characteristic peak at 570 cm−1 is covered up by other strong peaks in Fig. 3(d).
O stretching vibrations of the –COOH groups. Several absorption bands in the region of 1500–1200 cm−1 were assigned to the O–H deformations of the C–OH groups and stretching vibrations. The absorption peaks at 720 and 1969 cm−1 represent SO3− and R3NH+, respectively.36 Because the intensity of these polar groups is relatively strong, the Fe–O characteristic peak at 570 cm−1 is covered up by other strong peaks in Fig. 3(d).
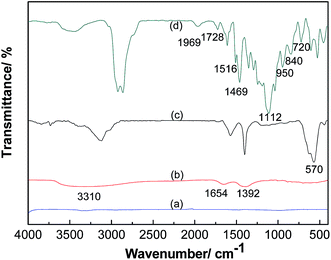 | ||
| Fig. 3 FTIR curves of (a) pristine MWCNT, (b) carboxylic MWCNT (c) MWCNT/Fe3O4 (d) the liquid-like MWCNT derivative. | ||
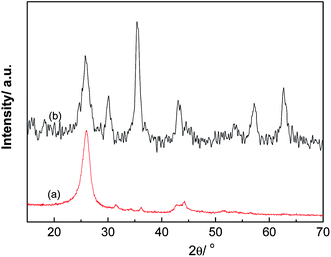
The XRD patterns of MWCNT and MWCNT/Fe3O4 are shown in Fig. 4. According to the MWCNT standard card (41-1487) and the Fe3O4 standard card (19-0629), it can be indicated that for MWCNT (Fig. 4(a)), the diffraction peaks at 2θ = 26.04°, 43.8° are assigned to (002), (100) planes of MWCNT. These two diffraction peaks are attributed to the graphitic structure of MWCNT.37 For MWCNT/Fe3O4, the (220), (331), (222), (511), (440) planes of Fe3O4 were observed at 2θ = 30.06°, 35.36°, 43.14°, 57.2°, 62.72°, respectively. The characteristic peaks of MWCNT still exist in Fig. 4(b), indicating that the graphitic structure of MWCNT is not destroyed after MWCNTs are coated with Fe3O4 nanoparticles.
The TEM images of the liquid-like MWCNT derivative are shown in Fig. 5. Compared with the pristine MWCNTs (Fig. 5(a)), the carboxylic MWCNTs (Fig. 5(b)) are shorter, suggesting that the carboxylation procedure can incise the MWCNTs effectively. It can be observed that in the liquid-like MWCNT derivative, the Fe3O4 nanoparticles are attached onto the MWCNT effectively (Fig. 5(c) and (e)). The size of the Fe3O4 nanoparticles ranges from 8 to 12 nm.
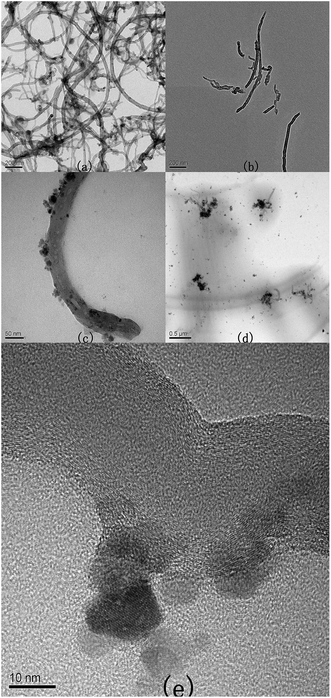 | ||
| Fig. 5 TEM pictures of (a) pristine MWCNT, (b) carboxylic MWCNT, (c and d) and (e) the liquid-like MWCNT derivative. | ||
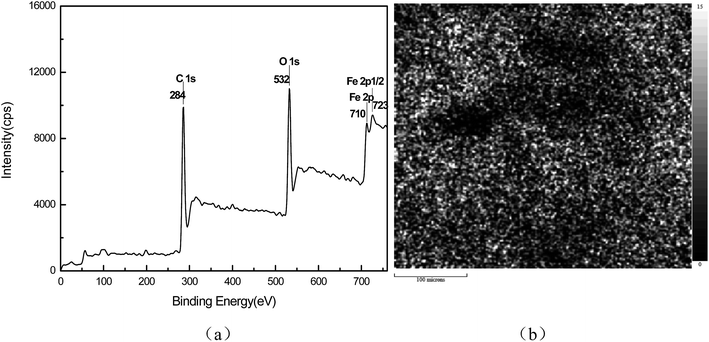
The XPS curve of MWCNT/Fe3O4 is shown in Fig. 6(a), in which there were different atoms, including the C1s photoelectron at 284 eV, the O1s photoelectron at 532 eV, the Fe2p photoelectron at 710 eV and the Fe2p1/2 photoelectron at 723 eV. The XPS imaging for Fe is shown in Fig. 6(b). The small spot areas were Fe, which are distributed in the hybrid system. It can be seen that the MWCNT are encrusted with Fe3O4 nanoparticles.
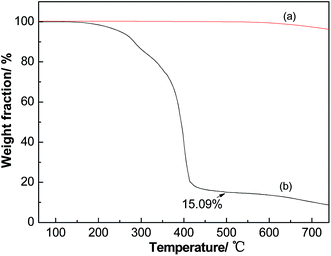
TGA curves of the liquid-like MWCNT derivative (Fig. 7(b)) showed almost no weight loss at the temperature below 150 °C. This indicates that the sample is nearly void of any conventional solvent such as water and acetone. The weight loss above 150 °C is due to the decomposition of the surfactants. Decomposition of MWCNT starts from 580 °C (Fig. 8(a)). The content of the inorganic component (MWCNT and Fe3O4) in the liquid-like MWCNT derivative is about 15.09 wt%.
There are many –CH2CH2O– (EO) units in PEGS, which can crystallize at low temperature. The DSC trace (Fig. 8(b)) of the PEGS shows a large exotherm at −5.21 °C and a endotherm at 23.93 °C, corresponding to crystallization and melting of PEGS, respectively. As for the liquid-like MWCNT derivative, the crystallizing temperature decreases to −39.97 °C and the crystallization heat is 16.56 J g−1. This indicates that crystallinity percentage decreases from 100% of PEGS to 55.1% of the liquid-like MWCNT derivative. The possible reason is that MWCNTs confine the crystal of the EO unit. Therefore, the crystalline structure of the MWCNT derivative is damaged, and the melting temperature of the MWCNT derivative decreases. Rodriguez38 and Warren39 reported a similar conclusion.
Rheological property of the liquid-like MWCNT derivative
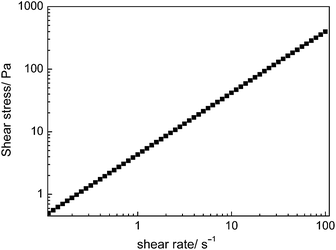
The steady flow test result (Fig. 9) showed that the MWCNT derivative exhibited Newtonian behaviour. It agreed with the work of Chen and Prasher, which reported Newtonian behaviour of EG/TiO2 (ref. 40) and propylene glycol/Al2O3 (ref. 41) nanoparticle-based fluids.The viscosity of the liquid-like MWCNT derivative decreased dramatically with the increasing temperature (Fig. 10). To be exact, the viscosity decreases from 3.87 Pa s (25.7 °C) to 0.37 Pa s (80 °C). This feature makes it possible for future application of the liquid-like MWCNT derivative in fabricating MWCNT/polymer nanocomposites. When the temperature is 60.7 °C, the viscosity of the liquid-like MWCNT derivative is 0.75 Pa s. However, this viscosity is so low that the liquid-like MWCNT derivative can disperse homogeneously in the polymer matrix. As a result, the processability of the nanocomposites is improved remarkably.
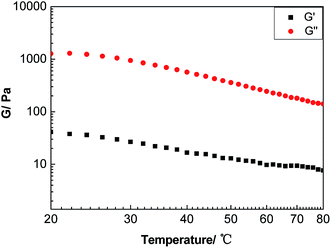
The storage modulus (G′) denotes the elastic behavior of materials, which is the driving force for molecule deformation. The loss modulus (G′′) represents the consumption energy of viscous deformation for materials. Because the liquid materials have permanent deformation due to flow and exhibit viscous behavior, G′′ is higher than G′. Fig. 11 shows that the liquid-like MWCNT derivative shared the same characteristic with liquid material since G′′ was always higher than G′ over the temperature range from 20 °C to 80 °C.
By grafting organic materials onto the surface of the MWCNT/Fe3O4, the solubility characteristic of the MWCNT derivative was changed. Fig. 12 and 13 show the solubility of the liquid-like MWCNT derivative in solvent after stewing for 24 h and 10 days. The concentration of the solution is 10 mg mL−1. Note that MWCNTs are nearly insoluble in all organic solvents. However, the liquid-like MWCNTs derivative is soluble in good solvent of PEGS such as acetone (Fig. 13(b)) and dichloroethane (Fig. 13(c)). The two solutions are quite stable. No large-scale agglomeration was observed after 10 days, and the derivative is slightly soluble in water (Fig. 13(a)). However, large-scale agglomeration was observed after 5 days, and unfortunately the derivative is still insoluble in ether (Fig. 12(g)) even in short term. Note that large-scale agglomeration was observed after 10 minutes probably because ether is not a good solvent for the surfactant that we used.
 | ||
| Fig. 12 Solubility of the liquid-like MWCNT derivative in (a) water, (b) acetone, (c) dichloroethane, (d) methanol, (e) tetrahydrofuran, (f) methylbenzene and (g) ether after 1 day. | ||
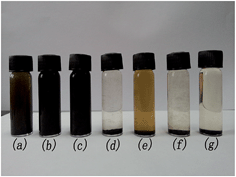 | ||
| Fig. 13 Solubility of the liquid-like MWCNT derivative in (a) water, (b) acetone, (c) dichloroethane, (d) methanol, (e) tetrahydrofuran, (f) methylbenzene and (g) ether after 10 days. | ||
Dispersion of the liquid-like MWCNT derivative in solvent and polymer matrix
One of the crucial challenges for nanoparticle research is to improve the dispersion of nanoparticles in solvents and polymer matrix. Fig. 5(d) shows that the MWCNT derivative homogeneously disperses in ethanol. The weight fraction of the MWCNT derivative in the MWCNT derivative/ethanol solution is 1%. Moreover, TEM images of the MWCNT derivative/epoxy nanocomposite (Fig. 14) show that the MWCNT derivative disperse homogeneously in the epoxy matrix. The weight fraction of the liquid-like MWCNT derivative is 1% and the curing agent is METHPA.It is highly possible that the better dispersion of the liquid-like MWCNT derivative in epoxy is due to the long and flexible organic surfactant shell. The canopy of the liquid-like MWCNT derivative, which was made up by PEGS, consists of many EO units. These EO units can improve compatibility of the liquid-like MWCNT derivative in ethanol and the epoxy matrix. Moreover, the long and flexible organic surfactant shell can bridge MWCNTs and make it harder for MWCNTs to entangle.
Property of MWCNT derivative/epoxy nanocomposites
Since the MWCNT derivative can disperse well in the epoxy matrix, the mechanical and thermal property of MWCNT derivative/epoxy nanocomposites may improve significantly. Therefore, impact toughness and Tg of MWCNT derivative/epoxy nanocomposites were investigated. According to Fig. 15, when the content of the MWCNT derivative is 1 wt%, the liquid-like MWCNT derivative can improve the impact toughness of epoxy by 194%. However, for comparision, adding only solid MWCNT can improve the impact toughness of epoxy by 51.7%. The liquid-like MWCNT derivative has an organic shell that consists of a long flexible chain, which can act as plasticiser and improve compatibility of nanoparticles and the matrix.42,43 The interfacial adhesion between the liquid-like MWCNT derivative and epoxy might be remarkably improved due to the organic shell, which has a hydrogen bond and reacts with the epoxy. Moreover, the dispersion of nanometer-sized particles in the polymer matrix has a significant impact on the mechanical properties of nanocomposites.44 Therefore, better dispersion of MWCNTs in epoxy is one of the reasons why impact toughness of epoxy resin improved.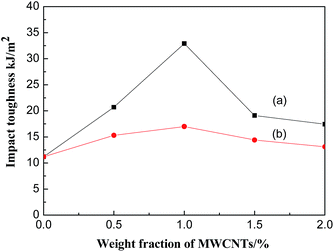 | ||
| Fig. 15 Impact toughness of (a) the liquid-like MWCNT derivative/epoxy nanocomposites, (b) MWCNT/epoxy nanocomposites. | ||
According to Fig. 16, the liquid-like MWCNT can improve the Tg of the epoxy by 16.9 °C. The result is quite impressive since traditional ways to improve toughness of a polymer (like adding plasticizer) can often deteriorate the thermal property of the polymer.45 However, the MWCNT derivative can improve the impact toughness and Tg simultaneously. The possible reason is that nanoparticles may react with the epoxy matrix and act as chemical crosslinking points. The hydroxyl and carboxyl groups on the surface of MWCNT and Fe3O4 nanoparticles can react with the epoxy matrix. Therefore, chemical crosslinking was formed and crosslink density was increased; consequently, the Tg of epoxy was improved.46
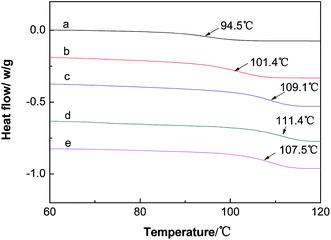 | ||
| Fig. 16 The DSC curves of liquid-like MWCNT derivative/epoxy nanocomposites with a MWCNT derivative loading of (a) 0%. (b) 0.5%, (c) 1%, (d) 1.5%, (e) 2%. | ||
Fig. 17 shows the DSC curve of the curing process of the nanocomposite. The curing temperature of the liquid-like MWCNT derivative/epoxy nanocomposite decreases slowly as the liquid-like MWCNT derivative loading increases. This is because there are a lot of hydroxyl groups in the derivative. The hydroxyl group can accelerate the curing process of epoxy. In this way, the liquid-like MWCNT derivative can act as an accelerant and decrease the curing temperature of the nanocomposite.
 | ||
| Fig. 17 DSC curves of the nanocomposite curing process. MWCNT derivative loading (a) 0%. (b) 0.5%, (c) 1%, (d) 1.5%. | ||
Magnetic property of the liquid-like MWCNT derivative
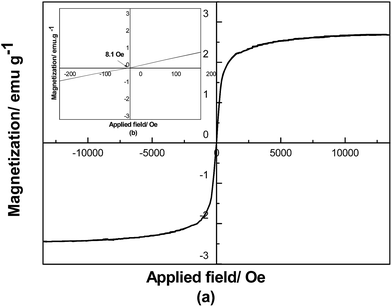
According to Fig. 18, the MWCNT derivative is a typical super-paramagnetic material. The specific magnetization of the MWCNT derivative is 2.68 emu g−1. This indicates that the MWCNT derivative can act as a special ferrofluid with PEGS as a carrier. The surfactant SID3392 is attached onto the surface of the MWCNT derivative through a covalent bond. There is electrostatic interaction between PEGS (carrier) and SID3392 (surfactant). The MWCNT derivative is quite stable, and no large scale agglomeration is observed after stewing for 3 months.Alignment of the liquid-like MWCNT derivative in solvent and polymer matrix
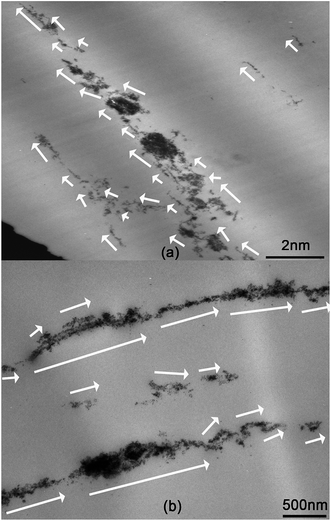
Since the MWCNT derivative is a magnetic material, it may be aligned in a bulk composite under a magnetic field. Fig. 19 shows alignment of the MWCNT derivative in the epoxy matrix. In this picture, each MWCNT derivative is marked by a white arrow. The shape, diameter and length of each MWCNT derivatives are different. However, all MWCNT derivatives aligned in a similar direction and some MWCNT derivatives connected end-to-end. The matrix is epoxy and the curing agent is 593. In the MWCNT derivative, MWCNT formed a magnetic rod since Fe3O4 nanoparticles were attached onto the surface of MWCNT. Therefore, the MWCNT derivative can be easily aligned to the magnetic field direction. Due to their magnetic susceptibility, the north and south poles of the MWCNT derivative show linear arrangement, which results in the end-to-end connectivity.Conclusions
A MWCNT derivative with liquid-like behaviour at room temperature was prepared by employing MWCNT/Fe3O4 as a core, tertiary amine-terminated organosilanes as a corona and sulfonic-acid as a canopy. The derivative was soluble in organic solvents and dispersed homogeneously in solvent and polymer matrix. Moreover, the derivative can improve impact toughness and heat resistance of epoxy simultaneously. Furthermore, the MWCNT derivative can be aligned in the epoxy matrix under a magnetic field. These advantages make it possible for us to develop a green and efficient route to fabricate high performance nanocomposite by using a liquid-like nanoparticle derivative. The super-paramagnetic nature of the MWCNT derivative may provide us a new way to manufacture ferrofluid. Our future study will focus on application of the liquid-like nanoparticle derivative in other promising fields like nanocatalysis and explore the fluidity mechanism of the liquid-like nanoparticle derivative.Acknowledgements
This work is supported financially by the National Natural Science Foundation (51373137, 51373136) and graduate starting seed fund of Northwestern Polytechnical University (Z2013164).References
- T. S. Jo, H. Han, L. Z. Ma and P. K. Bhowmik, Polym. Chem., 2011, 2, 1953–1955 RSC.
- M. E. Mackay, A. Tuteja, P. M. Duxbury, C. J. Hawker, B. V. Horn and Z. B. Guan, et al., Science, 2006, 311, 1740–1743 CrossRef CAS PubMed.
- W. D. Zhang, L. Shen, I. Y. Phang and T. X. Liu, Macromolecules, 2004, 37, 256–259 CrossRef CAS.
- K. I. Winey and R. Vaia, MRS Bull., 2011, 32, 314–322 CrossRef.
- P. Podsiadlo, A. K. Kaushik, E. M. Arruda, A. M. Waas, B. S. Shim and J. Xu, et al., Science, 2007, 318, 80–83 CrossRef CAS PubMed.
- P. Imin, F. Y. Cheng and A. Adronov, Polym. Chem., 2011, 2, 1404–1408 RSC.
- J. Z. Sun, A. J. Qin and B. Z. Tang, Polym. Chem., 2013, 4, 211–223 RSC.
- N. J. Fernandes, J. Akbarzadeh, H. Peterlik and E. P. Giannelis, ACS Nano, 2013, 7, 1265–1271 CrossRef CAS PubMed.
- T. Michinobu, T. Nakanishi, J. P. Hill, M. Funahashi and K. Ariga, J. Am. Chem. Soc., 2006, 128, 10384–10385 CrossRef CAS PubMed.
- J. X. Zhang, Y. P. Zheng, P. Y. Yu, S. Mo and R. M. Wang, Polymer, 2009, 50, 2953–2957 CrossRef CAS PubMed.
- Y. A. Lei, C. X. Xiong, H. Guo, J. L. Yao, L. J. Dong and X. H. Su, J. Am. Chem. Soc., 2008, 130, 3256–3257 CrossRef CAS PubMed.
- Y. A. Lei, C. X. Xiong and H. Guo, Small, 2007, 3, 1889–1893 CrossRef CAS PubMed.
- M. Robert, Q. T. Michael, M. Angelika, W. M. Christopher and B. Alexander, Chem. Sci., 2010, 1, 603–608 RSC.
- J. X. Zhang, Y. P. Zheng, P. Y. Yu, S. Mo and R. M. Wang, Carbon, 2009, 47, 2776–2781 CrossRef CAS PubMed.
- H. G. Li, J. Y. Choi and T. Nakanishi, Langmuir, 2013, 29, 5394–5406 CrossRef CAS PubMed.
- D. P. Liu, G. D. Li, Y. Su and J. S. Chen, Angew. Chem., Int. Ed., 2006, 45, 7530–7533 CrossRef.
- S. S. Babu, J. Aimi, H. Ozawa, N. Shirahata, A. Saeki and S. Seki, et al., Angew. Chem., Int. Ed., 2012, 51, 3391–3395 CrossRef PubMed.
- T. J. Kramer, S. S. Babu, A. Saeki, S. Seki, J. Aimi and T. Nakanishi, J. Mater. Chem., 2012, 22, 22370–22373 RSC.
- R. Rodriguez, R. Herrera, A. B. Bourlinos, R. P. Li, A. Amassian, L. A. Archer and E. P. Giannelis, Appl. Organomet. Chem., 2010, 24, 581–589 CrossRef CAS.
- T. Michinobu, K. Okoshi, Y. Murakami, K. Shigehara, K. Ariga and T. Nakanishi, Langmuir, 2013, 29, 5337–5344 CrossRef CAS PubMed.
- S. S. Babu, M. J. Hollamby, J. Aimi, H. Ozawa, A. Saeki and S. Seki, et al., Nat. Commun., 2013, 4, 1969 Search PubMed.
- M. M. H. Sadrabadi, E. Dashtimoghadam, F. S. Majedi, S. M. Wu, A. Bertsch and H. Moaddel, et al., RSC Adv., 2013, 3, 7337–7346 RSC.
- K. Yu, Y. J. Liu and J. S. Leng, RSC Adv., 2014, 4, 2961–2968 RSC.
- P. M. Ajayan, O. Stephan, C. Colliex and D. Trauth, Science, 1994, 265, 1212–1214 CAS.
- Z. Jin, K. P. Pramoda, G. Xu and S. H. Goh, Chem. Phys. Lett., 2001, 337, 43–47 CrossRef CAS.
- M. L. Auad, M. A. Mosiewicki, C. Uzunpinar and R. J. Williams, Polym. Eng. Sci., 2012, 50, 183–190 Search PubMed.
- D. Tasis, N. Tagmatarchis, A. Blanco and M. Prato, Chem. Rev., 2006, 106, 1105–1136 CrossRef CAS PubMed.
- Q. Li, L. J. Dong, L. B. Li, X. H. Su, H. Xie and C. X. Xiong, Carbon, 2012, 50, 2045–2060 CrossRef PubMed.
- Y. K. Yang, L. J. Yu, R. G. Peng, Y. L. Huang, C. E. He and H. Y. Liu, et al., Nanotechnology, 2012, 23, 225701 CrossRef PubMed.
- E. Camponeschi, R. Vance, M. A. Haik, H. Garmestani and R. Tannenbaum, Carbon, 2007, 45, 2037–2046 CrossRef CAS PubMed.
- E. L. Camponeschi, J. W. Walker, H. Garmestani and R. Tannenbaum, Microsc. Microanal., 2010, 15, 1060–1061 CrossRef.
- M. Abdalla, D. Dean, M. Theodore, J. Field, E. Nyairo and G. Price, Polymer, 2010, 51, 1614–1620 CrossRef CAS PubMed.
- A. Sharma, B. Tripathi and Y. K. Vijay, J. Membr. Sci., 2010, 361, 89–95 CrossRef CAS PubMed.
- A. B. Bourlinos, A. Bakandritsos, R. Zboril, M. Karakassides and C. Trapalis, Carbon, 2007, 45, 1105–1136 CrossRef PubMed.
- A. B. Bourlinos, V. Georgakilas, N. Boukos, P. Dallas, C. Trapalis and E. P. Giannelis, Carbon, 2007, 45, 1583–1595 CrossRef CAS PubMed.
- A. B. Bourlinos, V. Georgakilas, V. Tzitzios, N. Boukos, R. Herrera and E. P. Giannelis, Small, 2006, 2, 1188–1191 CrossRef CAS PubMed.
- X. J. Fan and X. Lin, New Carbon Mater., 2012, 27, 111–116 CrossRef CAS.
- R. Rodriguez, R. Herrera, A. B. Bourlinos, R. P. Li, A. Amassian and L. A. Archer, et al., Appl. Organomet. Chem., 2010, 24, 581–589 CrossRef CAS.
- S. C. Warren, M. J. Banholzer, L. S. Slaughter, E. P. Giannelis, F. J. DiSalvo and U. B. Wiesner, J. Am. Chem. Soc., 2006, 128, 12074–12075 CrossRef CAS PubMed.
- H. Chen, Y. Ding and C. Tan, Chem. Phys. Lett., 2007, 444, 333–337 CrossRef CAS PubMed.
- R. Prasher, D. Song, J. Wang and P. Phelan, Appl. Phys. Lett., 2006, 89, 133108 CrossRef PubMed.
- Y. P. Zheng, Y. Zheng and R. C. Ning, Mater. Lett., 2003, 57, 2940–2944 CrossRef CAS.
- J. H. Shi, B. X. Yang, K. P. Pramoda and S. H. Goh, Nanotechnology, 2007, 18, 375704 CrossRef.
- P. C. Ma, S. Y. Mo, B. Z. Tang and J. K. Kim, Carbon, 2012, 48, 1824–34 CrossRef PubMed.
- I. T. Kim, A. Tannenbaum and R. Tannenbaum, Carbon, 2011, 49, 54 CrossRef CAS PubMed.
- Y. P. Zheng, J. Northwest. Polytech. Univ., 2002, 20, 492–496 Search PubMed.
| This journal is © The Royal Society of Chemistry 2014 |