Fluorescent and antioxidant studies of effectively synthesized isochromenopyrrolone analogues
Hariyapureddy Raveendranatha Reddy,
Chitreddy V. Subba Reddy,
Radhakrishnan Subashini* and
Selvaraj Mohana Roopan*
Organic Chemistry Division, School of Advanced Sciences, VIT University, Vellore 632014, Tamilnadu, India. E-mail: mohanaroopan.s@gmail.com; dr.subashini.r@gmail.com; Fax: +91-416-224-3092; Tel: +0416-220-2352
First published on 17th June 2014
Abstract
An efficient strategy for the synthesis of 3-acetyl-2-methyl-1-phenylisochromeno [4,3-b] pyrrol-5(1H)-ones 4a–f have been developed using montmorillonite K10 as a catalyst. The reaction proceeded from acetyl acetone, substituted primary amines and ninhydrin via acidic media under solvent-free conditions. Compounds 4a–c exhibit green fluorescence under UV light, and their fluorescent properties in the liquid state were investigated. All the synthesized compounds were subjected for the evaluation of their free radical scavenging activity. Among all the tested compounds 4a and 4c exhibited good percentage inhibition in comparison with the standard, i.e. ascorbic acid.
1. Introduction
Aromatic heterocyclic compounds are known to show a wide range of ecotoxic effects, e.g. acute toxicity, cytotoxicity,1 photo-induced toxicity,2 mutagenicity3 and carcinogenicity.4 New efficient methods for the synthesis of heterocycles are a topic of major interest in modern synthetic organic chemistry.5 Among the heterocycles isocoumarin represents the foremost class of naturally occurring lactones that display a wide range of biological activities such as protease inhibitor properties, antifungal, cytotoxic, anti-allergic, antimicrobial and antimalarial effects.6–10 Isocoumarins are a class of natural products with a broad spectrum of biological activity,11,12 including antioxidative,13 antiangiogenic,14 antifungal,15,16 anti-allergic and antimicrobial8–10 activities. They are also important in medicinal chemistry as building blocks for bioactive compounds.17,18 In addition to its natural occurrence,19,20 various synthetic methodologies for construction of isocoumarins have been demonstrated.11 Note that isocoumarins are useful synthetic precursors to other heterocyclic and carbocyclic compounds.21,22Free radicals oxidatively damage lipids, proteins and genomic DNA integrity, and they are widely recognized as the root cause of numerous degenerative diseases including cancer.23 Antioxidants are potent scavengers of free radicals and serve as inhibitors of neoplastic processes.24 Vegetables, fruits and their seeds are rich sources of vitamins C, E, β-carotene and other compounds that might protect the organism against free radical induced injury and diseases.25,26 There has been an increasing interest in the use of natural antioxidants, such as tocopherols, flavonoids for the preservation of food materials.27 These natural antioxidants avoid the toxicity problems, which may arise from the use of synthetic antioxidants such as butylated hydroxy anisole (BHA), butylated hydroxy toluene (BHT) and propyl gallate (PG). Due to the complexity of antioxidant activity in foods, the evaluation of synthetic heterocyclic compounds has been widely focused on recently.28
In this paper, synthesis of isochromenopyrrolone analogues using MK-10 under solvent-free conditions as an approach for organic synthesis is described, which involves microwave exposure of the neat reactants. The synthesis of 3-acetyl-2-methyl-1-phenylisochromeno [4,3-b] pyrrol-5(1H)-ones 4a–f were carried out using MK-10 as a catalyst was found to be more efficient with high yields compared to conventional synthesis methods.29
2. Results and discussion
2.1 Chemistry
In this work, we have reported the synthesis of a novel series of 3-acetyl-2-methyl-1-phenylisochromeno [4,3-b] pyrrol-5(1H)-ones 4a–f using MK-10 as a catalyst. The antioxidant activity and fluorescence properties of these 3-acetyl-2-methyl-1-phenylisochromeno [4,3-b] pyrrol-5(1H)-ones 4a–f have also been reported.The formation of 4a–f was confirmed by FTIR, 1H NMR, 13C NMR and mass spectral analysis. FT-IR data of compound 4a shows an absorption peak at 1462 cm−1, indicating benzene C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching, a peak at 1668 cm−1, indicating alkene C
C stretching, a peak at 1668 cm−1, indicating alkene C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching, a peak at 1732 cm−1, indicating C
C stretching, a peak at 1732 cm−1, indicating C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching, and peaks at 3199–3057 cm−1, indicating sp3 C–H stretching. 1H-NMR data of compound 4a confirms the existence of one methyl of pyrrole ring, one methyl group of the acetyl group and two methyl groups of substituted amine groups at δ 1.04, 1.04, 2.32, 2.79 ppm respectively, as well as two methylene protons of the ethyl groups at δ 2.26 and the seven aromatic protons that exist between δ 6.2 to 8.36 ppm. 13C-NMR data of compound 4a confirms the existence of six aliphatic carbons at δ 12.26–31.30 ppm, sixteen aromatic carbons between δ 110.4–142.1 ppm and two carbonyl groups at δ 161.99 (–C
O stretching, and peaks at 3199–3057 cm−1, indicating sp3 C–H stretching. 1H-NMR data of compound 4a confirms the existence of one methyl of pyrrole ring, one methyl group of the acetyl group and two methyl groups of substituted amine groups at δ 1.04, 1.04, 2.32, 2.79 ppm respectively, as well as two methylene protons of the ethyl groups at δ 2.26 and the seven aromatic protons that exist between δ 6.2 to 8.36 ppm. 13C-NMR data of compound 4a confirms the existence of six aliphatic carbons at δ 12.26–31.30 ppm, sixteen aromatic carbons between δ 110.4–142.1 ppm and two carbonyl groups at δ 161.99 (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of isocoumarin ring) and 193.66 (–C
O of isocoumarin ring) and 193.66 (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of the acetyl group) (Scheme 1).
O of the acetyl group) (Scheme 1).
2.2 Fluorescence studies
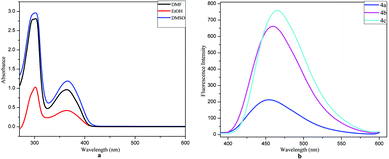
For the fluorescence studies, we found that the compound 4c exhibits a strong emission at 464 nm, the compound 4b shows emission at 462 nm, which is similar to that of 4c, and the compound 4a shows weak emission at 456 nm.2.3 Hydrogen peroxide scavenging assay
In the present study, hydrogen peroxide scavenging assay was performed for isochromenopyrrolones 4a–f. The absorbance of the standard (ascorbic acid) and the compounds 4a–f was measured at 230 nm, and the results were tabulated in Table 1. The percentage inhibition of the target molecules 4a–f has been plotted against the various concentrations of the standard (ascorbic acid) and the test solutions (Fig. 1). All readings were obtained in triplicate to check their accuracy.| Conc. μL | % Inhibition | |||||
|---|---|---|---|---|---|---|
| 4a | 4b | 4c | 4d | 4e | 4f | |
| 10 | 41.63 | 40.62 | 40.81 | 11.07 | 22.61 | 10.54 |
| 20 | 50.85 | 45.68 | 48.14 | 20.84 | 31.74 | 18.19 |
| 30 | 58.38 | 53.64 | 54.76 | 32.42 | 48.89 | 32.16 |
| 50 | 84.46 | 83.82 | 74.05 | 52.68 | 72.14 | 52.05 |
| 100 | 88.70 | 87.64 | 83.64 | 76.21 | 82.38 | 74.89 |
| IC50 | 1.84 | 2.07 | 2.07 | 2.46 | 2.90 | 2.38 |
 | ||
| Fig. 1 Comparison of the percentage inhibition of H2O2 assay of 3-acetyl-2-methyl-1-phenylisochromeno [4,3-b] pyrrol-5(1H)-ones 4a–f. | ||
2.4 DPPH assay
In the present study, the DPPH assay was performed for the isochromenopyrrolones 4a–f. The absorbance of the standard (ascorbic acid), and compounds 4a–f were measured at 520 nm, and the results are listed in Table 2. The percentage inhibition of the target molecules have been plotted against the various concentrations of the standard (ascorbic acid) and the test solutions 4a–f (Fig. 2). All readings were obtained in triplicate to check their accuracy.| Conc. μL | % Inhibition | ||||||
|---|---|---|---|---|---|---|---|
| 4a | 4b | 4c | 4d | 4e | 4f | 4a | |
| 10 | 24.96 | 21.62 | 22.13 | 18.70 | 17.28 | 19.15 | 16.78 |
| 50 | 55.73 | 51.89 | 50.20 | 54.00 | 48.55 | 52.26 | 41.51 |
| 100 | 92.77 | 88.15 | 85.73 | 86.92 | 67.35 | 85.09 | 64.42 |
| IC50 | 1.76 | 1.88 | 1.91 | 1.90 | 2.22 | 1.93 | 2.38 |
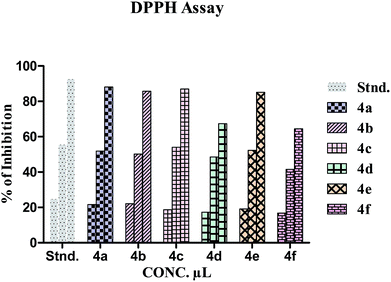 | ||
| Fig. 2 Comparison of the percentage inhibition of DPPH assay of 3-acetyl-2-methyl-1-phenylisochromeno [4,3-b] pyrrol-5(1H)-ones 4a–f. | ||
3. Experimental
Solvents and reagents were commercially sourced and used without further purification. Thin-layered chromatography (TLC) was performed on preparative plates of silica gel. Visualization was made using a UV chamber. Column chromatography was performed by using silica gel (60–120 mesh). Melting points were measured on a Elchem Microprocessor-based DT apparatus using an open capillary tube, and they are corrected with the standard, i.e. benzoic acid. IR spectra were obtained on a Nucon infrared spectrophotometer (KBr disc). UV-vis spectrum was obtained on UV-2550, Shimadzu Corporation, Kyoto, Japan. The NMR spectrum was recorded on a Bruker Avance III- 400 & 500 MHz spectrometer with tetramethylsilane (TMS) as an internal standard. Chemical shifts were reported in δ units (ppm). GC-MS spectra were recorded on a Perkin Elmer spectrophotometer, GC model: clarus 680, mass spectrometer model: clarus 600 (EI). The fluorescence spectra were obtained on Hitachi F-7000 FL spectrophotometer.3.1 General procedure for the synthesis of 3-acetyl-2-methyl-1-phenylisochromeno [4,3-b] pyrrol-5(1H)-one (4f)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) to afford the crystals of intermediate compound 3f.
8) to afford the crystals of intermediate compound 3f.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) as the eluent to afford the reddish brown crystals of isochromenopyrrolone 4a.
2) as the eluent to afford the reddish brown crystals of isochromenopyrrolone 4a.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) to afford the crystals of compound 4f. Optimization of 3-acetyl-2-methyl-1-phenylisochromeno [4,3-b]pyrrol-5(1H)-one 4a–f was performed by comparing the yield of the conventional and microwave irradiation methods. The results of the comparison are listed in Table 3.
8) to afford the crystals of compound 4f. Optimization of 3-acetyl-2-methyl-1-phenylisochromeno [4,3-b]pyrrol-5(1H)-one 4a–f was performed by comparing the yield of the conventional and microwave irradiation methods. The results of the comparison are listed in Table 3.
| Entry | Product | Time | Yield (%) | M.P. (°C) | ||
|---|---|---|---|---|---|---|
| CHa (h) | MWb (min) | CHa | MWb | |||
| a Conventional heating–stirring, 85 °C, gla. AcOH, conc. H2SO4.b Microwave irradiated, MK-10. | ||||||
| 1 | 4a | 2.45 | 1.20 | 79 | 82 | 209 |
| 2 | 4b | 2.40 | 1.10 | 78 | 84 | 202 |
| 3 | 4c | 2.50 | 1.40 | 86 | 95 | 18029 |
| 4 | 4d | 2.45 | 1.20 | 83 | 88 | 187 |
| 5 | 4e | 2.45 | 1 | 85 | 89 | 194 |
| 6 | 4f | 3.05 | 1.10 | 82 | 85 | 24529 |
3.2 Antioxidant assay
| % of RSC (%) = [(Ab − As)/Ab] × 100 |
RSC = radical scavenging capacity
As = absorbance of sample
Ab = absorbance of blank
| DPPH˙ + RH = DPPH + R˙ |
The DPPH radical scavenging activities of all the synthesized compounds 4a–f and ascorbic acid were determined according to the reported method.31 Initially, 1 mg mL−1 of the test material solutions and the standard (ascorbic acid) at a concentration of 10, 50 and 100 μg mL−1, respectively, were added to 1 mL of 0.2 mM freshly prepared solution of DPPH in ethanol. The reaction mixture was stirred vigorously and allowed to stand for 30 min at room temperature under dark conditions. The absorbance of each reaction mixture was measured at 520 nm. The percentage of DPPH radical scavenging activity (%) of the sample was calculated as follows:
| % (Inhibition) = [(Ab − As)/Ab] × 100 |
Ab = absorption of blank sample
As = absorption of sample
3.3 Fluorescence studies
Conclusions
In the present study, we report an efficient one-pot MK-10-catalysed synthesis of fused isochromenopyrrole-5(1H)-ones, which gave an enhanced yield with reduced time compared to conventional heating methods. Among the synthesized compounds, 4c and 4b exhibits strong fluorescence emission. The synthesized compounds were successfully screened for the free radical scavenging activity by H2O2 and DPPH assay method. Compounds 4a, 4b and 4c showed a high percentage of inhibition similar to that of the standard, i.e. ascorbic acid.Notes and references
- R. Rossi, A. Carpita, F. Bellina, P. Stabile and L. Mannina, Tetrahedron, 2003, 59, 2067–2081 CrossRef CAS.
- E. B. Rimm, A. Ascherio, E. Grovannucci, D. Spielgelman, W. J. Stampfer and W. C. Willett, J. Am. Med. Assoc., 1996, 275, 447–451 CrossRef CAS.
- I. Amin, M. M. Zamaliah and W. F. Chin, Food Chem., 2004, 87, 581–586 CrossRef PubMed.
- K. A. Steinmetz and J. D. Potter, J. Am. Diet. Assoc., 1996, 96, 1027–1039 CrossRef CAS.
- S. M. Roopan and F. N. Khan, Med. Chem. Res., 2011, 20, 732–737 CrossRef CAS.
- K. M. Meepagala, G. Sturtz and D. E. Wedge, J. Agric. Food Chem., 2002, 50, 6989–6992 CrossRef CAS PubMed.
- A. C. Whyte, J. B. Gloer, J. A. Scott, D. Malloch and A. C. Cercophorins, J. Nat. Prod., 1996, 59, 765–769 CrossRef CAS PubMed.
- J. C. Powers, J. L. Asgian, O. D. Ekici and K. E. James, Chem. Rev., 2002, 102, 4639–4750 CrossRef CAS PubMed.
- M. Yoshikawa, E. Harada, Y. Naitoh, K. Inoue, H. Matsuda, H. Shimoda, J. Yamahara and N. Murakami, Chem. Pharm. Bull., 1994, 42, 2225–2230 CrossRef CAS.
- K. F. Devienne, M. S. G. Raddi, R. G. Coelho and W. Vilegas, Phytomedicine, 2005, 12, 378–381 CrossRef CAS PubMed.
- R. D. Barry, Chem. Rev., 1964, 64, 229–260 CrossRef CAS.
- R. S. Mali and K. N. Babu, J. Org. Chem., 1998, 63, 2488–2492 CrossRef CAS PubMed.
- K. F. Devienne, A. F. C. Helena, D. J. Dorta, I. M. R. Prado, M. S. G. Raddi, W. Vilegas, S. A. Uyemura, A. C. Santos and C. Curti, Phytochemistry, 2007, 68, 1075–1080 CrossRef CAS PubMed.
- J. H. Lee, Y. J. Park, H. S. Kim, Y. S. Hong, K. W. Kim and J. J. Lee, J. Antibiot., 2001, 54, 463–466 CrossRef CAS.
- D. Engelmeier, F. Hadacek, O. Hofer, G. Lutz-Kutschera, M. Nagl, G. Wurz and H. Greger, J. Nat. Prod., 2004, 67, 19–25 CrossRef CAS PubMed.
- K. Nozawa, M. Yamada, Y. Tsuda, K. Kawai and S. Nakajima, Chem. Pharm. Bull., 1989, 29, 3486–3493 CrossRef.
- T. K. Devon and A. I. Scott, Handbook of Naturally Occurring Compounds, Academic Press, New York, 1975, vol. 1 Search PubMed.
- R. C. Elderfield, Heterocyclic Compounds, Wiley, New York, 1951, vol. 2 Search PubMed.
- W. Zhang, K. Krohn, S. Draeger and B. Schulz, J. Nat. Prod., 2008, 71, 1078–1081 CrossRef CAS PubMed.
- X. Lu, D. Li, N. K. Dalley, S. G. Wood and N. L. Owen, Nat. Prod. Res., 2007, 21, 677–685 CrossRef CAS PubMed.
- C. D. Gabbutt, J. D. Hepworth, B. M. Heron and J.-L. Thomas, Chem. Commun., 1999, 6, 541–542 RSC.
- E. Napolitano, Org. Prep. Proced. Int., 1997, 29, 631–664 CrossRef CAS.
- O. I. Aruoma, Food Chem. Toxicol., 1994, 32, 671–683 CrossRef CAS.
- D. Bagchi, A. Garg, R. L. Krohn, M. Bagchi, M. X. Tran and S. J. Stohs, Res. Commun. Mol. Pathol. Pharmacol., 1997, 95, 179–189 CAS.
- H. Tapiero, K. D. Tew, G. N. Ba and G. Mathe, Biomed. Pharmacother., 2002, 56, 200–207 CrossRef CAS.
- J. B. Harborne and C. A. Williams, Phytochemistry, 2000, 55, 481–504 CrossRef CAS.
- K. J. Hunter and J. M. Fletcher, Innovative Food Sci. Emerging Technol., 2002, 3, 399–406 CrossRef CAS.
- I. Hinneburg, H. J. Dorman and R. Hiltunen, Food Chem., 2006, 97, 122–129 CrossRef CAS PubMed.
- S. Pathak, A. Kundu and A. Pramanik, Tetrahedron Lett., 2011, 52, 5180–5183 CrossRef CAS PubMed.
- G. K. Jayaprakasha, L. Jaganmohan Rao and K. K. Sakariah, Bioorg. Med. Chem., 2004, 12, 5141–5146 CrossRef CAS PubMed.
- R. G. O. Rumbaoa, D. F. Cornago and I. M. Geronimo, J. Food Compos. Anal., 2009, 22, 546–550 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |