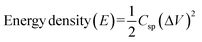Polyaniline-wrapped 1D CoMoO4·0.75H2O nanorods as electrode materials for supercapacitor energy storage applications†
Manas Mandala,
Debasis Ghosha,
Soumen Giria,
Imran Shakirb and
Chapal Kumar Das*a
aMaterials Science Centre, Indian Institute of Technology Kharagpur, Kharagpur – 721302, India. E-mail: chapal12@yahoo.co.in
bSustainable Energy Technologies (SET) Centre, College of Engineering, King Saud University, PO Box 800, Riyadh 11421, Kingdom of Saudi Arabia
First published on 19th June 2014
Abstract
In this study, a simple and cost effective one-pot hydrothermal process has been carried out for the synthesis of 1D CoMoO4·0.75H2O nanorods. A binary composite of CoMoO4·0.75H2O/PANI has also been synthesized by the in situ oxidative polymerization of aniline with virgin CoMoO4·0.75H2O. Two types of PANI morphologies have been demonstrated: amorphous nanodimensional PANI uniformly coated on CoMoO4·0.75H2O nanorods, and interconnected hollow spheres like PANI inside the bulk material. The prepared CoMoO4·0.75H2O/PANI composite was characterized by X-ray diffraction analysis and Fourier transform infrared spectroscopy for the phase and formation, respectively. The surface morphology was investigated by using FESEM and TEM, which revealed the formation of the CoMoO4·0.75H2O/PANI composite. The electrochemical characterization of the pseudocapacitive CoMoO4·0.75H2O and CoMoO4·0.75H2O/PANI composites in 1 M Na2SO4 showed the highest specific capacitances of 285 F g−1 and 380 F g−1, respectively, at a current density of 1 A g−1. The cyclic stability test demonstrated the specific capacitance retention of about 90.4% after 1000 consecutive charge–discharge cycles at a constant current density of 1 A g−1, which is also higher than that of virgin CoMoO4·0.75H2O—86.3% retention of specific capacitance.
Introduction
Recently, the synthesis and design of novel materials for energy storage devices as an alternative to the depleting traditional energy resources like fossil fuels has become a crucial and challenging topic for scientists. As a promising future for energy storage systems, supercapacitors, which are also called electrochemical capacitors, have attracted great significance due to their unique properties such as long operating lifetimes, high power density, moderated energy density, green environmental protection, and supporting the voltage of a system during enhanced loads from portable equipment to electric vehicles.1 Based on their energy storage mechanisms, supercapacitors are classified into two types: (i) electrochemical double layer capacitor (EDLC) and (ii) pseudocapacitor or redox supercapacitor, involving non-faradaic and faradaic processes, respectively.2 Non-faradaic processes do not involve any redox reactions; the energy is stored by the accumulation of ionic charges on the electrode/electrolyte interfaces. Carbonaceous materials, such as activated carbon, carbon nanotube, carbon aerogel, carbon cloth and graphene, are the most commonly used materials as electrodes in EDLC.3,4 In the faradaic process, energy is produced by the fast reversible faradaic transitions redox reactions of the active materials. Typical effective pseudocapacitive materials consist of transition metal oxides and hydroxides such as MnO2,5 Co(OH)2,6 RuO2,7 Ni(OH)2,8 NiO,9 and conducting polymers such as polyaniline,10 polypyrrole,11 and polythiophene.12 Among the transition metal oxides, RuO2 yields very high specific capacitance, but it is still not used commercially due to less availability and very high cost. However, the future growth in the field of supercapacitors is based on two approaches: either to develop a new material through the methods of solid-state chemistry/materials science or to improve the microstructure of existing materials. Moreover, binary metal oxides such as NiMoO4,13 CoMoO4,14 and MnMoO4 (ref. 15) have garnered significant research interest recently due to their feasible variable oxidation states and comparative higher electrical conductivity. Liu et al.14 reported a specific capacitance of 326 F g−1 for hydrothermally synthesized one-dimensional CoMoO4·0.9H2O nanorods at a current density of 5 mA cm−1. To improve the performance of CoMoO4, various efforts have been devoted such as the incorporation of carbonaceous materials like MWCNT16 or graphene.17 Xu et al. obtained a specific capacitance of 170 F g−1 for microwave-synthesized CoMoO4/MWCNTs at a current density of 0.1 A g−1.16 However, Xia et al. achieved a specific capacitance of about 394.5 F g−1 at the scan rate of 1 mV s−1 for hydrothermally synthesized CoMoO4/graphene composites.17Polyaniline (PANI), a unique conjugated polymer, has been extensively studied as a promising material for energy storage and conversion with its high conductivity, exhibiting a fast reversible faradaic reaction, excellent pseudocapacitive behavior and low cost, as well as ease of synthesis.18 The major problem with a virgin electrode of PANI is poor cycling stability as the active redox site of the polymer backbone is demolished within a limited number of charge-discharge cycles. Therefore, to increase the cyclic stability, PANI is often used as a hybrid material with metal oxide/sulfide or carbonaceous materials such as graphene and carbon nanotubes.19–22
In our present work, we have demonstrated an in situ oxidative polymerization method for the preparation of nanodimensional amorphous polyaniline coated 1D CoMoO4·0.75H2O nanorods for supercapacitor energy storage applications. The composite material can be effectively handled within a larger potential range exhibiting high energy density at a high power delivery rate. The synthesis and physical and electrochemical characterizations are discussed in detail.
Materials and method
All the materials were used without further purification. Aniline (99.5%) monomer and ammonium persulphate (APS) were purchased from Merck chemicals, India. Carbon black, polyvinylidene difluoride (PVDF) and sodium molybdate (Na2MoO4) were purchased from Sigma Aldrich Ltd., Germany. Cobalt chloride hexahydrate (CoCl2·6H2O) and ethanol were procured from Loba Chemie, India. All the chemicals used in this research were of analytical grade, and doubly distilled water was used throughout the experiment. All the aqueous solutions were prepared with doubly distilled water.Preparation of CoMoO4·0.75H2O
For the preparation of CoMoO4·0.75H2O, we used a facile hydrothermal method. In the typical synthesis procedure, 20 ml 0.1 M CoCl2·6H2O solution was mixed with 20 ml 0.1 M sodium molybdate (Na2MoO4) slowly with continuous stirring. The mixture was then transferred into a 50 ml capacity Teflon-sealed autoclave and heated in a muffle furnace at 180 °C for 16 h. A violet precipitate was obtained. The precipitates were centrifuged and washed with a water–ethanol mixture several times and dried at 60 °C. The as-prepared sample was labeled as CoMoO4·0.75H2O.Preparation of CoMoO4·0.75H2O/PANI composite
For the preparation of the CoMoO4·0.75H2O/PANI composite, an in situ oxidative polymerization process was followed, in which ammonium persulfate was used as the oxidant. A total of three reaction sets were prepared by varying the concentration of aniline monomer and the APS while the amount of CoMoO4·0.75H2O was kept constant. Briefly, the aniline monomer was well-dispersed in 100 ml ice water by ultrasonication for 5 min. Then, 150 mg of the prepared CoMoO4·0.75H2O was added to it and stirred for two minutes at a low rpm. APS was dissolved in 100 ml ice water and the solution was poured drop by drop to the above suspension of the prepared CoMoO4·0.75H2O containing aniline monomer with continuous stirring at 250 rpm and the stirring was continued for 3 h. The temperature of the bulk material was kept at 0–5 °C during the whole stirring process. Then, the whole material was kept in a refrigerator overnight, and then washed with water and ethanol several times and the final precipitation was dried at 60 °C. The amount of APS used were 0.5 g, 1 g and 1.5 g for 0.1 ml, 0.2 ml and 0.3 ml aniline monomer, respectively, and the prepared materials were labeled as CoMoO4·0.75H2O/PANI (0.1), CoMoO4·0.75H2O/PANI (0.2), and CoMoO4·0.75H2O/PANI (0.3). Pure PANI was prepared by using 0.2 ml aniline monomer and 1 g APS as the oxidant.Results and discussions
Materials characterizations
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching of quinonoid rings and C
C stretching of quinonoid rings and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching of benzenoid rings, respectively. The absorption peak at 1291 cm−1 corresponds to C–N stretching.26 The band at 3400 cm−1 indicates the stretching of the N–H band of the aromatic ring in PANI and CoMoO4·0.75H2O/PANI. The peaks at 1112 cm−1 and 802 cm−1 are attributed to the characteristic of the C–N
C stretching of benzenoid rings, respectively. The absorption peak at 1291 cm−1 corresponds to C–N stretching.26 The band at 3400 cm−1 indicates the stretching of the N–H band of the aromatic ring in PANI and CoMoO4·0.75H2O/PANI. The peaks at 1112 cm−1 and 802 cm−1 are attributed to the characteristic of the C–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond stretching27 and the out-of-plane bending vibration of C–H bond in benzene rings, respectively.26 For the CoMoO4·0.75H2O/PANI composite, all the absorption peaks are similar to the absorption peaks of PANI, except the peak at 951 cm−1 for Mo–O stretching, which is shifted to the right side by a marginal extent in the composite, suggesting that the composite surface is composed of PANI.28
C bond stretching27 and the out-of-plane bending vibration of C–H bond in benzene rings, respectively.26 For the CoMoO4·0.75H2O/PANI composite, all the absorption peaks are similar to the absorption peaks of PANI, except the peak at 951 cm−1 for Mo–O stretching, which is shifted to the right side by a marginal extent in the composite, suggesting that the composite surface is composed of PANI.28
Morphological analysis
The morphological analysis of the materials was performed in terms of FESEM and TEM analysis, and the images are shown in Fig. 3 and 4, respectively. Fig. 3a and b show the FESEM images of CoMoO4·0.75H2O nanorods at various magnifications, revealing its average diameter of 200–500 nm with an average length of 2–5 μm. The FESEM images of the binary composites using different concentrations of aniline monomer of 0.1 ml, 0.2 ml and 0.3 ml are shown in Fig. 3c, d and f, respectively. The FESEM image of the binary composites prepared using 0.1 ml aniline monomer indicate the presence of nanospherical PANI coated over the CoMoO4 nanorods. However, a nonuniform coating of PANI was observed due to the presence of the low amount of PANI. The surface morphology of the CoMoO4·0.75H2O/PANI composite using 0.2 ml aniline indicates that the nanorod architecture of CoMoO4·0.75H2O is almost buried under PANI (Fig. 3d), and an individual CoMoO4·0.75H2O nanorod was hardly found. This indicates an excellent coating of amorphous PANI on the nanorods. Fig. 3e shows the higher magnification image of the composite, revealing the sphere-like morphology of PANI in bulk. Fig. 3f indicates that the size of PANI spheres increases with the amount of PANI from 0.2 ml to 0.3 ml. The elemental mapping of the CoMoO4·0.75H2O/PANI composite prepared using 0.2 ml aniline is shown in Fig. S1 (see ESI),† which indicates the presence of Co, Mo, and O for CoMoO4·0.75H2O as well as C and N for PANI in the CoMoO4·0.75H2O/PANI composite. The corresponding weight and atomic percentages of the elements are shown in Fig. S2.† The EDX analysis also confirms the successful formation of the CoMoO4·0.75H2O/PANI composite in which the nanorods are buried under the PANI.The TEM image (Fig. 4a) of CoMoO4·0.75H2O also suggests its rod-like morphology, and the corresponding SAED pattern indicates its single crystalline nature (Fig. 4b). Fig. 4c and d are the TEM images of the CoMoO4·0.75H2O/PANI composite prepared using 0.2 ml PANI at low and high magnifications, respectively. These images also support the FESEM analysis. In case of the binary composite, two types of PANI morphologies can be seen: amorphous nanodimensional PANI uniformly coated on CoMoO4·0.75H2O nanorods and interconnected hollow spheres like PANI (Fig. 4e) with a diameter of about 0.8–1 μm inside the bulk material.
Electrochemical characterizations
For the electrochemical measurements, we used a three electrode system, where active materials fabricated on nickel foam (1 cm × 1 cm), Pt electrode and saturated calomel electrode were selected as the working electrode, counter electrode and reference electrode, respectively. For the preparation of the working electrode, active materials, carbon black and polyvinylidene fluoride (PVDF) were taken in N-methyl-2-pyrrolidone (NMP) with the ratio of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The prepared paste was cast onto nickel foam and allowed to dry fully under air. The electrochemical tests, galvanostatic charge–discharge (GCD) and cyclic voltammetry (CV) techniques were performed by using a Biologic SP-150 instrument in an aqueous 1 M Na2SO4 electrolyte.
1. The prepared paste was cast onto nickel foam and allowed to dry fully under air. The electrochemical tests, galvanostatic charge–discharge (GCD) and cyclic voltammetry (CV) techniques were performed by using a Biologic SP-150 instrument in an aqueous 1 M Na2SO4 electrolyte.
Fig. 5a and b demonstrate the cyclic voltammetry plots of virgin CoMoO4·0.75H2O nanorod and pure PANI at different scan rates of 2, 10 and 20 mV s−1, respectively. The CV plots of virgin CoMoO4·0.75H2O have a pair of redox peaks during the positive and negative sweeps, suggesting that the specific capacitance arises from the redox mechanism. The faradaic reactions corresponding to the redox peaks are attributed to the redox reaction of Co(II)/Co(III).29 The well-defined redox peaks at a high scan rate (20 mV s−1) indicate that the materials have the properties of high rate capability and good reversibility.
 | ||
| Fig. 5 Cyclic voltammetry curves of CoMoO4·0.75H2O (a) and pure PANI (b) at different scan rates of 2, 10 and 20 mV s−1. | ||
The specific capacitance (CS in F g−1) of the materials from the CV measurement can be calculated by using the following equation.
 determines the area of the I–V curve.
determines the area of the I–V curve.
The highest specific capacitance of CoMoO4·0.75H2O and pure PANI are 373 F g−1 and 306 F g−1 at a scan rate of 2 mV s−1, respectively (Table 1).
| Scan rates | 2 mV s−1 | 10 mV s−1 | 20 mV s−1 |
|---|---|---|---|
| Sp. cap. (F g−1) of CoMoO4·0.75H2O | 373 | 287 | 254 |
| Sp. cap. (F g−1) of pure PANI | 306 | 256 | 212 |
The CoMoO4·0.75H2O/PANI composite shows larger symmetry and curve area due to increasing conductivity and pseudocapacitive properties in the presence of the conducting polymer PANI. At different scan rates, there is no peak in the CV curves, indicating that the electrode material is charged and discharged at a pseudo-constant rate over the complete voltammetric cycle. By increasing the scan rate, the redox current increases, the anodic peak shifted towards the positive potential and the cathodic peak shifted towards the negative potential. The current response with the scan rate indicates the interfacial faradaic redox reactions and the rates of electronic/ionic transportations.29
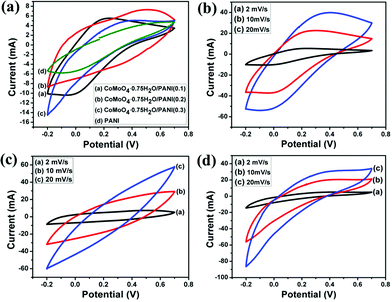
To understand the optimum content ratio of CoMoO4·0.75H2O nanorod to aniline monomer in the composite for the best electrochemical behaviour, cyclic voltammetry analysis of all the three CoMoO4·0.75H2O/PANI composites were carried out at different scan rates of 2, 10 and 20 mV s−1 and the plots are shown in Fig. 6. Amongst the three composites, the maximum specific capacitance of 475 F g−1 is exhibited by CoMoO4·0.75H2O/PANI (0.2). This specific capacitance value is much higher than the specific capacitance of 394.5 F g−1 at a scan rate of 2 mV s−1 for CoMoO4/graphene composites, as reported by Xia et al.17 Padmanathan et al.30 reported the specific capacitances of 450.2 F g−1 and 333.3 F g−1 for the hybrid CoMoO4/carbon and CoMoO4/reduced graphene oxide nanorods at a scan rate of 2 mV s−1, respectively, which are lower than that of the CoMoO4·0.75H2O/PANI (0.2) composite. For increasing scan rates, the specific capacitance of the electrode material decreases due to the slow redox reaction at a higher scan rate. The CoMoO4·0.75H2O/PANI composite using 0.2 ml aniline monomer shows the maximum specific capacitance amongst the three composites. The synergistic combination of both metal oxide and polyaniline plays a crucial role in the enhancement of the specific capacitance for CoMoO4·0.75H2O/PANI (0.2 ml) as compared to the other composites. It serves more redox active sites of polyaniline and metal oxide. This optimum aniline monomer (0.2 ml) leads to more accessibility of electrolyte, which ensures better accumulation of the charge and larger insertion/deinsertion of ions into/out of the composites during charging–discharging. In the case of other composites like CoMoO4·0.75H2O/PANI (0.1 ml), the PANI unit could not entirely cover the metal oxide homogeneously, which results in lesser redox active sites as compared with CoMoO4·0.75H2O/PANI (0.2 ml). On the other hand, the PANI unit for CoMoO4·0.75H2O/PANI (0.3 ml) is more densely attached with the metal oxide so that the electrolyte cannot penetrate the metal oxide wall, i.e. metal oxide cannot take part in the redox reaction, resulting in a low specific capacitance. Furthermore, a sharp current drop between +0.1 to −0.2 V is not the desirable CV behaviour of supercapacitor materials. The cyclic voltammetry curves of the CoMoO4·0.75H2O/PANI composite at different concentrations of aniline monomer of 0.1 ml, 0.2 ml, and 0.3 ml and pure PANI at a scan rate of 2 mV s−1 are shown in Fig. 8a.
The CoMoO4·0.75H2O/PANI composite prepared by using 0.2 ml aniline monomer shows the highest specific capacitance from the CV; therefore, the other electrochemical characterizations, such as galvanostatic charge–discharge measurements and electrochemical impedance spectroscopy study, were carried out using the CoMoO4·0.75H2O/PANI (0.2) composite (Table 2).
| Scan rates | 2 mV s−1 | 10 mV s−1 | 20 mV s−1 |
|---|---|---|---|
| Sp. cap. (F g−1) of CoMoO4·0.75H2O/PANI (0.1) | 459 | 361 | 284 |
| Sp. cap. (F g−1) of CoMoO4·0.75H2O/PANI (0.2) | 475 | 380 | 303 |
| Sp. cap. (F g−1) of CoMoO4·0.75H2O/PANI (0.3) | 442 | 349 | 267 |
In order to investigate the current–voltage behavior of the CoMoO4·0.75H2O and CoMoO4·0.75H2O/PANI composites materials, the coulombic efficiency was first measured to fix the appropriate potential window for these materials. The potential windows of (−0.2 to 0.4 V) for virgin CoMoO4·0.75H2O and (−0.2 to 0.7 V) for CoMoO4·0.75H2O/PANI composite were selected to obtain the best working efficiency. The coulombic efficiency was calculated from the galvanostatic charge–discharge experiments as follows:
The galvanostatic charge–discharge (GCD) curves of the CoMoO4·0.75H2O and CoMoO4·0.75H2O/PANI composites at various current densities of 1 A g−1, 2 A g−1 and 3 A g−1 are shown in Fig. 7c and d. The semi-symmetric GCD curves for both the materials reveal their pseudocapacitive behavior. The incorporation of PANI increases the charging–discharging time in the case of CoMoO4·0.75H2O/PANI composites.
 | ||
| Fig. 7 Galvanostatic charge–discharge curves of CoMoO4·0.75H2O (a) and CoMoO4·0.75H2O/PANI (b) at different current densities of 1, 2 and 3 A g−1. | ||
The specific capacitance (Csp) was calculated from the equation below:
| Current densities | 1 A g−1 | 2 A g−1 | 3 A g−1 |
|---|---|---|---|
| Sp. cap. (F g−1) of CoMoO4·0.75H2O | 285 | 163 | 133 |
| Sp. cap. (F g−1) of CoMoO4·0.75H2O/PANI | 380 | 237 | 213 |
The energy density and power density determine the fate of a material to behave as an electrode material in supercapacitor applications. The energy densities and power densities of the materials were calculated by the following equations:
where Csp is the specific capacitance in F g−1, ΔV is the potential window in V, and T is the discharge time of the charge–discharge curves from where the specific capacitance was calculated. Fig. 8c shows the energy density versus power density curves in terms of the Ragone plot. The highest energy density of 14.25 W h kg−1 and 42.7 W h kg−1 were achieved by the virgin CoMoO4·0.75H2O and CoMoO4·0.75H2O/PANI composites at a power density of 300 W kg−1 and 450 W kg−1, respectively. The CoMoO4·0.75H2O and CoMoO4·0.75H2O/PANI composites show comparable values of energy density and power density with some other similar composites, such as PANI/metal oxide and graphene/metal oxide composites. Zou et al.31 and Hu et al.32 reported the energy densities of 33.6 W h kg−1 and 42.4 W h kg−1 for WO3/PANI and PANI/SnO2 composites, respectively. Jaidev et al.19 revealed the maximum energy density of 17.8 W h kg−1 for the PANI/MnO2 hybrid nanocomposite. Xia et al.17 achieved the highest energy density of 54.8 W h kg−1 at a power density of 197.2 W kg−1 for the CoMoO4/graphene composite, which is higher than that of pure-CoMoO4 (10.0 W h kg−1 at a power density of 36.0 W kg−1) at a 1 mV s−1 scan rate. Ghosh et al.13 reported the energy densities of 4.444 and 10.833 W h kg−1 for NiMoO4·nH2O and graphene-NiMoO4·nH2O composites, respectively, at the same power density of 1250 W kg−1. The variation of the energy density and power density with current densities of both the materials is shown in Table 4. To determine the cycle stability, GCD was performed up to 1000 cycles at a current density of 1 A g−1. The cycle stability of the CoMoO4·0.75H2O and CoMoO4·0.75H2O/PANI composites are demonstrated in Fig. 8b. The retention of the specific capacitance of the CoMoO4·0.75H2O and CoMoO4·0.75H2O/PANI composites are 86.3% and 90.4%, respectively. The composite shows higher retention of the specific capacitance than the virgin electrode due to the synergistic effect between pure CoMoO4·0.75H2O and PANI. The low cycle stability of CoMoO4·0.75H2O can be explained by the swelling of the electrode materials through the faradaic reactions. In the case of these composites, the retention of the specific capacitance is only 4.1% higher than the virgin CoMoO4·0.75H2O composite: this can be explained by considering the deterioration of PANI after several shrinking and swelling charge–discharge cycles.
| Current densities | 1 A g−1 | 2 A g−1 | 3 A g−1 |
|---|---|---|---|
| Energy density (W h kg−1) of CoMoO4·0.75H2O | 14.25 | 8.2 | 6.65 |
| Power density (W kg−1) of CoMoO4·0.75H2O | 300 | 600 | 900 |
| Energy density (W h kg−1) of CoMoO4·0.75H2O/PANI | 42.7 | 26.6 | 23.87 |
| Power density (W kg−1) of CoMoO4·0.75H2O/PANI | 450 | 900 | 1350 |
To measure the efficiency of the electrode material, an electrochemical impedance spectroscopy (EIS) study was performed within the frequency range of 100 kHz to 10 MHz. Fig. 9 demonstrates the EIS of the pure CoMoO4·0.75H2O and CoMoO4·0.75H2O/PANI composites. The EIS has been represented in terms of a Nyquist plot that reveals electrochemical information such as charge transfer resistance, and solution resistance. The diameter of the semicircle represents the charge transfer electrochemical reaction resistance, also known as the faradaic resistance, and determines the rate of response of the electrode material in a particular electrolyte. From Fig. 9, it can be found that the semicircle diameter of virgin CoMoO4·0.75H2O (28 Ω) is higher than the diameter of the semicircle of the composite (6 Ω), suggesting that virgin CoMoO4·0.75H2O exhibits high resistance/low conductivity than the composite. These results indicate that the CoMoO4·0.75H2O/PANI composite is a more potential candidate as an electrode material for supercapacitor applications rather than virgin CoMoO4·0.75H2O.
Conclusions
In conclusion, the virgin CoMoO4·0.75H2O and CoMoO4·0.75H2O/PANI composites using different concentrations of aniline monomer were successfully synthesized by hydrothermal and in situ oxidative polymerization methods. Due to the presence of a variable oxidation state of the metal forming the metal molybdate compound, it can effectively behave as pseudocapacitive electrode material. A maximum specific capacitance of 285 F g−1 was achieved at 1 A g−1 current density accompanied with high energy density of 14.25 W h kg−1 at a power density of 300 W kg−1. The synergistic interaction of PANI with CoMoO4·0.75H2O resulted in an increased specific capacitance of 380 F g−1 and the larger working potential resulted in an elevated energy density of 42.7 W h kg−1 at a high power density of 450 W kg−1 accompanied with a better cycle life. The reasonably high value of energy density and power density along with the excellent cycling stability makes the CoMoO4·0.75H2O/PANI composite an efficient supercapacitor electrode material.Acknowledgements
The authors thank IIT Kharagpur, India for financial support and instrumental help. Authors are also thankful to Ms. Krishna Chattopadhyay, IIT Kharagpur, India, for the XRD analysis.References
- M. Zhu, C. J. Weber, Y. Yang, M. Konuma, U. Starke, K. Kern and A. M. Bittner, Carbon, 2008, 46, 1829–1840 CrossRef CAS PubMed.
- B. Conway, Electrochemical Supercapacitors, Kluwer Academic/Plenum Publishers, New York, 2nd edn, 1999 Search PubMed.
- L. L. Zhang and X. S. Zhao, Chem. Soc. Rev., 2009, 38, 2520–2531 RSC.
- Y. B. Tan and J.-M. Lee, J. Mater. Chem. A, 2013, 1, 14814–14843 CAS.
- B. Ming, J. Li, F. Kang, G. Pang, Y. Zhang, L. Chen, J. Xu and X. Wang, J. Power Sources, 2012, 198, 428–431 CrossRef CAS PubMed.
- D. Ghosh, S. Giri and C. K. Das, ACS Sustainable Chem. Eng., 2013, 1, 1135–1142 CrossRef CAS.
- V. Subramanian, S. C. Hall, P. H. Smith and B. Rambabu, Solid State Ionics, 2004, 175, 511–515 CrossRef CAS PubMed.
- D. Ghosh, S. Giri, A. Mandal and C. K. Das, Chem. Phys. Lett., 2013, 573, 41–47 CrossRef CAS PubMed.
- C. Yuan, X. Zhang, L. Su, B. Gao and L. Shen, J. Mater. Chem., 2009, 19, 5772–5777 RSC.
- D. Ghosh, S. Giri, A. Mandal and C. K. Das, Appl. Surf. Sci., 2013, 276, 120–128 CrossRef CAS PubMed.
- L.-Z. Fan and J. Maier, Electrochem. Commun., 2006, 8, 937–940 CrossRef CAS PubMed.
- A. Laforgue, P. Simon, C. Sarrazin and J.-F. Fauvarque, J. Power Sources, 1999, 80, 142–148 CrossRef CAS.
- D. Ghosh, S. Giri and C. K. Das, Nanoscale, 2013, 5, 10428–10437 RSC.
- M.-C. Liu, L.-B. Kong, X.-J. Ma, C. Lu, X.-M. Li, Y.-C. Luo and L. Kang, New J. Chem., 2012, 36, 1713–1716 RSC.
- K. K. Purushothaman, M. Cuba and G. Muralidharan, Mater. Res. Bull., 2012, 47, 3348–3351 CrossRef CAS PubMed.
- Z. Xu, Z. Li, X. Tan, C. M. B. Holt, L. Zhang, B. S. Amirkhiz and D. Mitlin, RSC Adv., 2012, 2, 2753–2755 RSC.
- X. Xia, W. Lei, Q. Hao, W. Wang and X. Wang, Electrochim. Acta, 2013, 99, 253–261 CrossRef CAS PubMed.
- H. Mi, X. Zhang, S. Yang, X. Ye and J. Luo, Mater. Chem. Phys., 2008, 112, 127–131 CrossRef CAS PubMed.
- R. I. Jafri, A. K. Mishra and S. Ramaprabhu, J. Mater. Chem., 2011, 21, 17601 RSC.
- K.-J. Huang, L. Wang, Y.-J. Liu, H.-B. Wang, Y.-M. Liu and L.-L. Wang, Electrochim. Acta, 2013, 109, 587–594 CrossRef CAS PubMed.
- Q. Wu, Y. Xu, Z. Yao, A. Liu and G. Shi, ACS Nano, 2010, 4, 1963–1970 CrossRef CAS PubMed.
- V. Gupta and N. Miura, Electrochim. Acta, 2006, 52, 1721–1726 CrossRef CAS PubMed.
- K. Eda, Y. Uno, N. Nagai, N. Sotani and M. S. Whittingham, J. Solid State Chem., 2005, 178, 2791–2797 CrossRef CAS PubMed.
- L. Ding, Q. Li, D. Zhou, H. Cui, H. An and J. Zhai, J. Electroanal. Chem., 2012, 668, 44–50 CrossRef CAS PubMed.
- G. Kianpour, M. S. Niasari and H. Emadi, Superlattices Microstruct., 2013, 58, 120–129 CrossRef CAS PubMed.
- C. Li, J. Wang, Y. Wen, Y. Ning, Y. Wen, X. Yuan, M. Li and D. Yang, ECS Electrochem. Lett., 2013, 2(1), H1–H4 CrossRef CAS PubMed.
- B. Kavitha, K. Prabakar, K. S. kumar, D. Srinivasu, Ch. Srinivas, V. K. Aswal, V. Siriguri and N. Narsimlu, IOSR J. Appl. Chem., 2012, 2, 16–19 CrossRef CAS PubMed.
- S. Kumar and S. Jain, J. Chem., 2014, 837682, DOI:10.1155/2014/837682.
- L.-Q. Mai, F. Yang, Y.-L. Zhao, X. Xu, L. Xu and Y.-Z. Luo, Nat. Commun., 2011, 2, 381–385 CrossRef PubMed.
- N. Padmanathan, K. M. Razeeb and S. Selladurai, Ionics, 2014 DOI:10.1007/s11581-014-1089-0.
- B.-X. Zou, Y. Liang, X.-X. Liu, D. Diamond and K.-T. Lau, J. Power Sources, 2011, 196, 4842–4848 CrossRef CAS PubMed.
- Z.-A. Hu, Y.-L. Xie, Y.-X. Wang, L.-P. Mo, Y.-Y. Yang and Z.-Y. Zhang, Mater. Chem. Phys., 2009, 114, 990 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: EDX and elemental mapping of CoMoO4·0.75H2O/PANI composite by using 0.2 ml aniline monomer. See DOI: 10.1039/c4ra03399j |
| This journal is © The Royal Society of Chemistry 2014 |