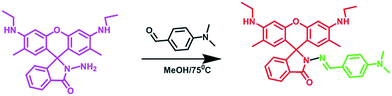
Rhodamine based selective turn-on sensing of picric acid†
Gandhi Sivaraman*,
Balasubramanian Vidya and
Duraisamy Chellappa*
School of chemistry, Madurai Kamaraj University, Madurai-625021, India. E-mail: raman474@gmail.com; dcmku123@gmail.com
First published on 2nd July 2014
Abstract
A rhodamine based derivative RDD-1 bearing dimethylaminobenzaldehyde has been designed and synthesized. It is a highly sensitive and selective chemosensor towards picric acid without having any interference from other NAC's. To the best of our knowledge, the present probe is the first example of a rhodamine based sensor that facilitates the detection of powerful explosive picric acid even at picomolar concentration.
Introduction
Chemosensors have fascinated scientists as they not only aid environmental remediation but also serve as diagnostic tools in the medicinal field. Extensive studies have therefore been undertaken towards developing probes to sense biologically relevant metal ions Cu2+, Zn2+, heavy metal ions Hg2+, Pb2+, and anions like F−, pyrophosphate ion and so on. Nevertheless, relatively little work has been done on sensing of explosives. Among various explosives, in early years picric acid (PA), due to its ability to withstand the shock of firing in conventional artillery,1 was widely used as explosive; used on small scale as antiseptic and for the treatment of burns, malaria and smallpox. Apart from this, it finds application in dye industries, pharmaceuticals and chemical laboratories.2 However, PA leads to respiratory problems3 by damage of organs, and cancer.4 The explosive and toxic nature of PA commands devising a suitable sensor for its detection. Various techniques such as gas chromatography, ion mobility spectrometry, Raman spectroscopy, fluorescence spectroscopy5 have been adopted for the detection of nitro explosives. Fluorescence is one of the preferred methods over others because of its high sensitivity, selectivity, fast response and easy sample preparation.6 Numerous fluorescent probes, though available for detection of picric acid and nitro-aromatics such as pyrene and poly cyclic aromatic derivatives, conjugated polymers, organic dye molecules, porphyrin derivatives etc.,7 suffer from background interference due to turn-off nature. Very recently Pang et al.,8a has registered for the first time ratiometric sensing of PA with a dansyl modified squaric acid dye. Recently Ravikanth et al.,8b have developed BODIPY based turn-on fluorescent chemodosimeter for picric acid. Development of rhodamine based sensors for various analytes in our laboratory prompted us to conceive the probe RDD-1. Generally turn-on fluorescent sensors are more preferred than turn-off fluorescent chemosensors, due to the turn-off detection affected by background fluorescence, which leads to low sensitivity.9 Rhodamine dyes having better photostability and visible excitation, emission maxima.10 Rhodamine derivatives are non-fluorescent at basic/neutral conditions; they exist in the spirocyclic form whereas rhodamine derivatives are fluorescent under acidic conditions, as the ring-opened form leads.11 It is well known that rhodamine 6G derivatives are much more sensitive than rhodamine B dye derivatives as fluorescent turn-on probes due to the steric hindrance provided by the methyl group which facilitates spirolactam ring opening process.12 The photonic study of the probe RDD-1 brings to light its capability of sensing PA through hydrogen bonding. To the best of our knowledge, this is the third example of turn-on fluorescence for the detection of PA.Results and discussion
Addition of rhodamine 6G hydrazide with 4-N,N-dimethylaminobenzaldehyde in ethanol under refluxing conditions yields the formation of the sensing probe RDD-1 (Scheme 1) with 78% yield. The probe RDD-1 was fully characterised by 1H, 13C-NMR, ESI-MS and elemental analysis. (Fig. S1 to S3 in ESI†) The probe RDD-1 in its electronic spectrum does not exhibit any absorption peak in visible region.During addition of PA, an absorption peak emerged at 535 nm. This absorption change in visible region makes feasible the colorimetric detection of PA by conspicuous colour change from colourless to pink. The appearance of a new absorption band at 535 nm is attributable to its spiroring-opened nature of the probe RDD-1. To check the selectivity, the photonics of Probe RDD-1 were investigated in the presence of few aromatic and nonaromatic compounds such as TNT, TNB, nitrobenzene, 2,4-dinitrophenol, benzoquinone etc. by UV-vis spectroscopy (Fig. 1).
 | ||
| Fig. 1 UV-visible absorption responses of RDD-1(5 μM) toward different concentrations of PA in water (0–10 equivalents). | ||
The absence of colour change reveals that the probe RDD-1 is selectively sensing PA. Although strong acids such as TFA, HCl produced an absorbance change at 527 nm but PA addition leads to band at 535 and 425 nm. The change in the absorbance wavelength makes feasible to distinguish PA from other strong acids (Fig. S11†).
The fluorescence spectrum of RDD-1 (1 μM) exhibits weak emission band at 541 nm when excited at 480 nm. The addition of PA to the solution of RDD-1 results in appearance of fluorescence emission at 557 nm with high intensity along with the concomitant decrease of fluorescence at 541 nm (Fig. 2 and 3). A linear relationship was obtained when a plot of fluorescence intensity vs. concentration of picric acid was constructed. It provides that the probe RDD-1 will be applicable for detection of picric acid (Fig. S7†). The RDD-1, when monitored with the addition of compounds like TNT, TNB, nitrobenzene, 2,4-dinitrophenol, benzoquinone etc., did not exhibit any change in its photonics.
 | ||
| Fig. 2 Fluorescence spectra of RDD-1 in the presence of various NAC's (PA, BQ, NB, NT, NP, DNB, TNT, DNP) in aqueous solution (excitation at 480 nm, slit width = 5 nm/5 nm). | ||
 | ||
| Fig. 3 Fluorescence responses of RDD-1 (5 μM) toward different concentrations of PA in aqueous (0–10 equivalents) solution. | ||
This, in turn, ascertains the selective fluorimetric response of RDD-1 towards picric acid. To get an insight into the turn-on fluorescence recognition mechanism, the absorption and fluorescence spectral behaviours of the probe with the addition of trifluoroacetic acid (TFA) were analysed under identical conditions. Upon addition of TFA, an absorption maximum was observed at 527 nm whereas fluorescence was noticed at 547 nm. With PA addition two absorption maxima, one at 535 nm and another at 425 nm were observed while fluorescence was seen at 557 nm. Further the intensity of the peaks arising in both absorption and fluorescence spectra of RDD-1 by PA addition is higher compared to that surfacing with TFA addition. It is obvious that RDD-1 displays varied responses towards TFA and PA. This in turn advocates that the mechanisms involved in sensing TFA and PA by RDD-1 are different. It is envisaged that TFA being strong acid protonates the dimethylamino group of RDD-1. This protonation in turn triggers the opening of spirolactam ring to result in the observed photonic changes. It is noted that strong acids such as TFA, HCl and PA triggered different spectral changes for Rhodamine derivative, i.e., TFA, HCl induced a fluorescence at 547 nm, but PA showed ratiometric fluorescence enhancement at 557 nm. (Fig. S11 and S12 in ESI†) Exhibition of different photonic changes by RDD-1 towards PA sensing coupled with the observation of higher intense peaks designates that RDD-1 may associate with PA through intermolecular hydrogen bonding. This hydrogen bonding association between PA and RDD-1 is augmented by the observation of a peak in ESI-MS at m/z = 789.4 that corresponds to [RDD-1 + PA + H]+ (Fig. S6 in ESI†) and further supported Job's plot analysis (Fig. S5 in ESI†). Further to confirm the formation of picrate adduct, 1H-NMR study was undertaken. 1H-NMR of RDD-1 gives only a single peak corresponding to two methyl groups of dimethylamino group. 1H-NMR titration of RDD-1 with PA showed that these methyl groups at 2.83 ppm get shifted, and new peak at 8.6 ppm is observed upon the addition of PA which is characteristic of picric acid proton. Concomitantly in the 1H-NMR titration of RDD-1 with PA in CDCl3, the characteristic methyl peak of rhodamine spirolactam at 1.8 gets shifted and the NH broad band at 3.56 completely disappeared on subsequent addition of PA. These results further confirmed that the turn-on fluorescence response of RDD-1 to PA is ascribed to the opening of spirolactam and formation of picrate adduct (Fig. 4).
We also carried out pH studies and found that emission spectra of RDD-1 in aqueous solution. Further, the effect of PA added with RDD-1in different pH values was also studied. At acidic pH (1–4) the probe RDD-1 shows fluorescence which corresponds to the ring opened species and with the addition of PA to RDD-1, the emission intensity was not much affected. In the acidic pH range the emission intensity of PA added RDD-1 get decreased because of protonation of the RDD-1 in acidic pH leads to the fluorescence change. Hence PA detection by RDD-1 is affected by some extent in highly acidic pH (Fig. S13 in ESI†). The quick response of the probe RDD-1 with PA revealed from the fluorescence measurements in different time intervals (Fig. S8 ESI†).
To find if there is any interference in the detection of PA, the photonics of RDD-1 were monitored with the mixture of interfering compounds and PA. The competitive experiments clearly establish that there is no interference from the added components towards sensing PA. We also studied the response time of RDD-1 with PA; it is noted that the colour and fluorimetric change emerged very quickly after addition of PA. It implies that the probe RDD-1 is suitable for quick detection of picric acid. The detection limit (LOD) was found to be 45 nM (3α/S) signifying its feasibility to detect PA even at ultra-low level concentrations. The binding constant of PA-1 with RDD-1 was calculated using Benesi–Hildebrand equation; it is found to be 5.64 × 107 M−1. In order to study the mechanism of photophysical changes during addition of picric acid to RDD-1 density functional theory (DFT) calculations was carried out with the Gaussian 09 program13 with the B3LYP/6-311G basis sets. Geometries of RDD-1 and RDD-1 + PA adduct were initially optimized using above said basis sets. The absorption behaviour and the corresponding transitions are obtained from TDDFT calculations using above said basis sets. (Table S1 in ESI†) The charges on the N atom of dimethyl amino unit in RDD-1 and RDD-1 + PA adduct are found to be −0.628, −0.573 respectively. This significant reduction in the electron density on the N atom upon binding of the picric acid with RDD-1 indicates the significant charge transfer between PA and RDD-1. Also it is found that the charge on both secondary amine N atoms remain unchanged with the addition of PA. Frontier molecular orbital (FMO) analysis further conformed the internal charge transfer (ICT) process occurred after appendage of PA to RDD-1. In RDD-1 HOMO localised on dimethylaminobenzaldimine unit, LUMO spread over on dimethylaminobenzaldimine and partially on rhodamine-6G unit (Fig. 5).
 | ||
| Fig. 5 Frontier molecular orbitals of RDD-1 and RDD-1 + PA obtained from the DFT calculations using Gaussian 09 program. | ||
In RDD-1 + PA, HOMO appears over the π moiety of RDD-1 unit whereas LUMO spreads over picric acid. The calculated difference in energy gap of RDD-1 (3.586 eV) decreases significantly to 2.077 eV upon binding with PA. Thus, decrease in energy difference of RDD-1 + PA clearly points out the formation of stronger charge transfer complex of RDD-1 with PA. This also supports the red shift observed in the emission peak. Hence these results clearly point out the intermolecular charge transfer taking place between PA and RDD-1. The involvement of ICT in the emissive state is also confirmed by the observations of solvatochromism in variation of solvent polarity (Fig. S14 in ESI†). In order to demonstrate the practical applicability of RDD-1 for PA detection, a test strip was made by dip coating the solution of RDD-1 on to a filter paper and then subsequently drying in air. The test strips, when immersed into the various concentration of PA in acetonitrile–water mixture, undergo perceptible colour change to red; red fluorescence was noticed upon illumination with hand-held UV lamp (Fig. 6 and 7).
 | ||
| Fig. 6 Visible (top) colour changes and under UV-light (bottom) of test strips after the addition of picric acid. | ||
Conclusion
We have designed and synthesized a rhodamine based derivative RDD-1 as highly sensitive and selective chemosensor for picric acid. To the best of our knowledge, the probe RDD-1 is the first example of rhodamine based sensor that facilitates the detection of powerful explosive picric acid even at picomolar concentration. Apart from this it doesn't have any interference from other NAC's. Further using test strips coated with sensor RDD-1, the feasibility of detecting PA through colorimetric/fluorescent mode has been demonstrated.Experimental section
Synthesis of RDD-1
Rhodamine 6G hydrazide (1.09 mmol) and 4-N,N-dimethyl aminobenzaldehyde (1.09 mmol) were refluxed in 10 ml ethanol for about 6 hours in the presence of catalytic amount of acetic acid. The reaction mixture upon cooling to room temperature, had thrown out a colourless solid. The precipitated solid was then purified by column chromatography using dichloromethane–ethyl acetate (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to yield colorless solid. Probe: 1H-NMR (300 MHz, CDCl3): δ 8.28 (s, 1H), 8.00 (dd, J = 5.4, 3.3 Hz, 1H), 7.47–7.38 (m, 4H), 7.03 (dd, J = 5.7, 2.9 Hz, 1H), 6.55 (d, J = 8.8 Hz, 2H), 6.39 (s, 2H), 6.36 (s, 2H), 3.48 (s, 2H), 3.20 (dd, J = 14.1, 7.0 Hz, 4H), 2.93 (d, J = 5.2 Hz, 6H), 1.85 (s, 6H), 1.30 (t, J = 7.1 Hz, 6H).13C-NMR (75 MHz, CDCl3): 14.65, 16.55, 38.25, 40.09, 65.61, 96.65, 106.49, 111.38, 117.82, 123.01, 123.08, 123.39, 127.62, 127.92, 128.86, 128.93, 132.89, 147.33, 147.54, 151.05, 151.38, 152.28, 164.72. Elemental analysis: calculated: C, 75.11; H, 6.66; N, 12.51%; observed: C, 75.19; H, 6.71; N, 12.63%; MS (ESI): 560.4603 (M + H)+, calculated: 559.2946.
1) to yield colorless solid. Probe: 1H-NMR (300 MHz, CDCl3): δ 8.28 (s, 1H), 8.00 (dd, J = 5.4, 3.3 Hz, 1H), 7.47–7.38 (m, 4H), 7.03 (dd, J = 5.7, 2.9 Hz, 1H), 6.55 (d, J = 8.8 Hz, 2H), 6.39 (s, 2H), 6.36 (s, 2H), 3.48 (s, 2H), 3.20 (dd, J = 14.1, 7.0 Hz, 4H), 2.93 (d, J = 5.2 Hz, 6H), 1.85 (s, 6H), 1.30 (t, J = 7.1 Hz, 6H).13C-NMR (75 MHz, CDCl3): 14.65, 16.55, 38.25, 40.09, 65.61, 96.65, 106.49, 111.38, 117.82, 123.01, 123.08, 123.39, 127.62, 127.92, 128.86, 128.93, 132.89, 147.33, 147.54, 151.05, 151.38, 152.28, 164.72. Elemental analysis: calculated: C, 75.11; H, 6.66; N, 12.51%; observed: C, 75.19; H, 6.71; N, 12.63%; MS (ESI): 560.4603 (M + H)+, calculated: 559.2946.
Acknowledgements
G.S. is grateful to UGC for a research fellowship. G.S. and D.C. acknowledges DST-IRHPA, FIST, and PURSE for funding and instrumental facilities.Notes and references
- H. Muthurajan, R. Sivabalan, M. B. Talawar and S. N. Asthana, J. Hazard. Mater., 2004, 112, 17 CrossRef CAS PubMed.
- E. H. Volwiler, Ind. Eng. Chem., 1926, 18, 1336 CrossRef CAS.
- (a) Safety data sheet for picric acid, resource of National Institute of Health; ; (b) V. Pimienta, R. Etchenique and T. Buhse, J. Phys. Chem. A, 2001, 105, 10037 CrossRef CAS.
- J. Shen, J. Zhang and R. He, J. Hazard. Mater., 2009, 163, 1199 CrossRef CAS PubMed.
- Y. Salinas and S. Gil, Chem. Soc. Rev., 2012, 41, 1261 RSC.
- M. E. Germain and M. J. Knapp, Chem. Soc. Rev., 2009, 38, 2543 RSC.
- (a) V. Bhalla, M. Kumar, D. S. Shankar Rao and S. Krishna Prasad, ACS Appl. Mater. Interfaces, 2013, 5, 672–679 CrossRef CAS PubMed; (b) V. Vij, V. Bhalla and M. Kumar, ACS Appl. Mater. Interfaces, 2013, 5, 5373 CrossRef CAS PubMed; (c) G. He and Y. Fang, J. Mater. Chem., 2009, 19, 7347 RSC; (d) B. Roy, A. K. Bar, B. Gole and P. S. Mukherjee, J. Org. Chem., 2013, 78, 1306 CrossRef CAS PubMed; (e) S. Pramanik, V. Bhalla and M. Kumar, Anal. Chim. Acta, 2013, 793, 99–106 CrossRef CAS PubMed; (f) N. Dey, S. K. Samanta and S. Bhattacharya, ACS Appl. Mater. Interfaces, 2013, 5, 8394–8400 CrossRef CAS PubMed; (g) V. Sathish, K. L. Lu and S. Rajagopal, J. Phys. Chem. B, 2013, 117, 14358–14366 CrossRef CAS PubMed; (h) S. Barman, O. Blacque and K. Venkatesan, Chem. Commun., 2012, 48, 11127 RSC; (i) V. Bhalla, A. Gupta and M. Kumar, Org. Lett., 2012, 14, 3112 CrossRef CAS PubMed.
- (a) Y. Xu, S. Sun and Y. Pang, Chem. Commun., 2013, 49, 4764 RSC; (b) S. Madhu, A. Bandela and M. Ravikanth, RSC Adv., 2014, 4, 7120 RSC.
- (a) J. R. Lakowicz, Principles of Fluorescence Spectroscopy, Kluwer Academic, New York, 1999 Search PubMed; (b) A. BarbaBon and F. Sancenon, Chem. Commun., 2012, 48, 3000 RSC.
- (a) H. N. Kim, M. H. Lee, H. J. Kim, J. S. Kim and J. Yoon, Chem. Soc. Rev., 2008, 37, 1465 RSC; (b) X. Chen, T. Pradhan, F. Wang, J. S. Kim and J. Yoon, Chem. Rev., 2012, 112, 1910 CrossRef CAS PubMed; (c) M. Beija and J. M. G. Martinho, Chem. Soc. Rev., 2009, 38, 2410 RSC; (d) G. Sivaraman, T. Anand and D. Chellappa, Analyst, 2012, 137, 5881 RSC; (e) G. Sivaraman and D. Chellappa, J. Mater. Chem. B, 2013, 1, 57689 RSC; (f) G. Sivaraman, V. Sathiyaraja and D. Chellappa, J. Lumin., 2014, 145, 480 CrossRef CAS PubMed; (g) M. Iniya and D. Chellapa, RSC adv., 2014, 4, 25393 RSC; (h) T. Anand, G. Sivaraman and D. Chellappa, J. Photochem. Photobiol., A, 2014, 281, 47 CrossRef CAS PubMed.
- L. Yuan, W. Lin and Y. Feng, Org. Biomol. Chem., 2011, 9, 1723 CAS.
- Y. K. Yang, J. Lee, I. Shin and J. Tae, Org. Lett., 2009, 11, 859 CrossRef CAS PubMed.
- D. J. Fox, Gaussian 09, Revision A.02, Gaussian, Inc., Wallingford CT, 2009 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra02931c |
| This journal is © The Royal Society of Chemistry 2014 |