Synthesis of poly(butylene succinate) through oligomerization–cyclization–ROP route
Abstract
The preparation of cyclic butylene succinate lactone via catalytic depolymerization of poly(butylene succinate) oligomers (OPBS) and conversion to high-molecular weight poly(butylene succinate) (PBS) via ring-opening polymerization (ROP) is described. OPBS was first synthesized by two-stage melt polycondensation, purified and characterized by size exclusion chromatography (SEC). Then, it was depolymerized under reduced pressure in a glass oven and the volatile fraction (VF) was collected and characterized. The butylene succinate lactones obtained by intramolecular transesterification were characterized by nuclear magnetic resonance (1H NMR) and mass spectrometry (MS). Their further successful ROP polymerization within 24 hours afforded the desired PBS with 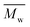 of 65 000 g mol−1 and Đ of 2.2.
of 65 000 g mol−1 and Đ of 2.2.


 Please wait while we load your content...
Please wait while we load your content...