Mg doping in nanosheet-based spherical structured ZnO photoanode for quasi-solid dye-sensitized solar cells
Abstract
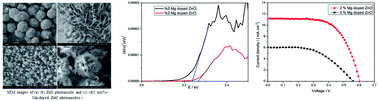
In this paper, Mg doping is utilized in the synthesis of a nanosheet-based spherical structured ZnO photoanode resulting in an increased overall efficiency from 1.72% to 4.19% of quasi-solid dye-sensitized solar cells. Existence of Mg in the ZnO crystal is detected by XRD and XPS measurements. The diameter of the ZnO nanosheet-based spherical structure is obviously decreased after Mg doping, which facilitates the fabrication of ZnO films and increases the specific area leading to larger dye loading and higher photocurrent. The energy bandgap of the ZnO photoanode with and without Mg doping is investigated by UV-vis spectra and theoretical calculation based on the GAUSSIAN 09 program and an increase of the bandgap is observed after Mg doping, which leads to an increase of the conduction band position and enhances the photo-voltage of DSSCs based on Mg doped ZnO photoanodes. An electrochemical impedance spectroscopy (EIS) test also indicates that DSSCs based on Mg doped ZnO show a strengthened effect of facilitating electron transport and retarding the charge recombination.

 Please wait while we load your content...
Please wait while we load your content...