Engineering of coloration responses of porous WO3 gasochromic films by ultraviolet irradiation
Abstract
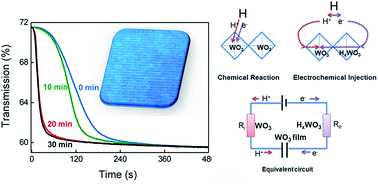
The coloration response of gasochromic films is crucial for gas sensors and solar energy cells. Based on a comparison of different post-treatments of WO3 gasochromic films, UV irradiation is found useful for fast coloration, in which fast exponential optical changes can be detected instead of a long activation delay process. Infrared spectroscopy, Raman spectroscopy, and X-ray photoelectron spectroscopy studies show that the gasochromic response of WO3 films depends on the ion and electron diffusion velocities, which can be engineered by altering the film porosities and conductivities. A new gasochromic model is proposed based on a resistor and capacitor (RC) circuit and the double-injection theory. According to this model, the slow coloration delay and fast exponential coloration should result from thermodynamic and electrochemical reactions, respectively. Our new model is also successfully used to build a link between two well-identified theoretical models.

 Please wait while we load your content...
Please wait while we load your content...