Simultaneous determination of dopamine, uric acid and ascorbic acid using a glassy carbon electrode modified with reduced graphene oxide
Abstract
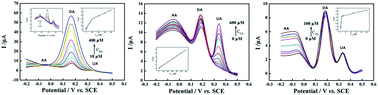
A facile and cost-effective approach has been developed towards electrochemical fabrication of a reduced graphene oxide (RGO) modified glassy carbon electrode (RGO/GCE). Scanning electron microscopy (SEM) images show that RGO is covered completely on the surface of glassy carbon electrodes. The RGO/GCE is used to detect dopamine (DA), uric acid (UA) and ascorbic acid (AA) simultaneously via cyclic voltammetry (CV) and differential pulse voltammetry (DPV) methods. Compared with bare GCE, RGO/GCE exhibits much high electrocatalytic activities toward the oxidation of DA, UA and AA, and three well-defined fully resolved anodic peaks were found in the CV curve at RGO/GCE. The GCEs modified with different amounts of RGO have an obvious influence on the determination of DA, UA and AA. By changing the concentrations of DA, UA and AA in the three substances coexisting system, the linear response ranges for the determination of DA, UA and AA were 0.1–400 μM, 2–600 μM, and 0.7–100 μM with the limit of detection (LOD) (S/N = 3) were estimated to be 0.1 μM, 1 μM and 0.7 μM, respectively. Moreover, it is found that RGO/GCE displays high reproducibility and selectivity for the determination of DA, UA and AA.

 Please wait while we load your content...
Please wait while we load your content...