Environmentally friendly solution route to kesterite Cu2ZnSn(S,Se)4 thin films for solar cell applications†
Abstract
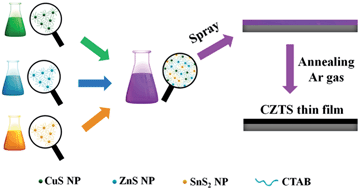
Quaternary kesterite Cu2ZnSnS4 (CZTS) and Cu2ZnSn(S,Se)4 (CZTSSe) thin films have been prepared from a mixture of CuS, ZnS and SnS2 nanoparticles and solar cells were made from the CZTSSe films. The binary sulfide nanoparticles were pre-synthesized in aqueous solution and then spray deposited onto glass substrates. The nano-sized binary sulfide nanoparticles have a large surface area that provides the driving force for solid-state reactions between the nanoparticles and results in the formation of the quaternary CZTS phase at moderate temperatures. The CZTSSe solar cells were prepared using the binary sulfide nanoparticles films annealed in Se vapor and the cells showed an encouraging efficiency of 5.12% (Voc = 378 mV, Jsc = 26.2 mA cm−2 and FF = 51.7%). Our synthetic approach provides a low-cost, environmentally friendly and easy to scale up option for the preparation of CZTSSe thin films for solar cell applications.

 Please wait while we load your content...
Please wait while we load your content...