Effects of template removal on both morphology of mesoporous silica-coated gold nanorod and its biomedical application
Abstract
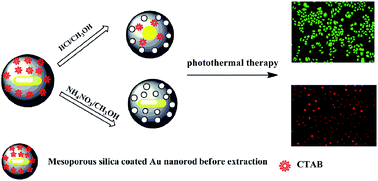
Mesoporous silica-coated Au nanorods (Aurod@SiO2) have recently attracted considerable interest in nanomedicine, and the template removal procedure is crucial for the preparation of such hybrid nanostructure. Herein, two kinds of typical extraction solvents, i.e., NH4NO3–CH3OH and HCl–CH3OH, were separately used to extract the surfactant cetyltrimethylammonium bromide (CTAB) from the obtained Aurod@SiO2. The results show that CTAB molecules could be completely removed from the pores of Aurod@SiO2 without damaging the internal Au nanorod by NH4NO3–CH3OH, while Aurod@SiO2 treated with HCl–CH3OH suffered from both poor extraction efficiency and the shape transformation of Au nanorod due to selective etching in the presence of oxygen. In addition, the consequent drug loading experiment shows that Aurod@SiO2 extracted by NH4NO3–CH3OH possesses a larger drug loading capacity with a loading efficiency of 82.5%. Furthermore, the in vitro photo-thermal therapy experiment shows that Aurod@SiO2 extracted by NH4NO3–CH3OH is more efficient in killing the YCC-2 gastric cancer cells as compared with that extracted by HCl–CH3OH.

 Please wait while we load your content...
Please wait while we load your content...