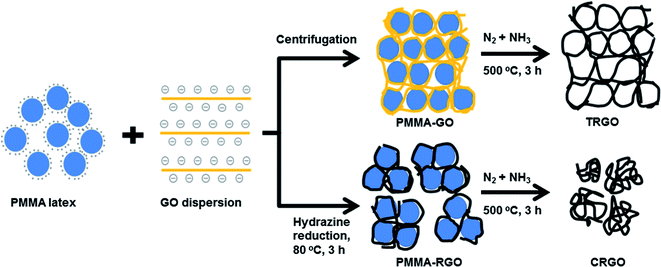
Nitrogen-doped mesoporous reduced graphene oxide for high-performance supercapacitors†
Viet Hung Phama,
Thuy-Duong Nguyen-Phana,
Jinhee Janga,
Thi Diem Tuyet Vua,
Yoon Jae Leeb,
In Kyu Songb,
Eun Woo Shina and
Jin Suk Chung*a
aSchool of Chemical Engineering and Bioengineering, University of Ulsan, Daehakro 93, Ulsan 680-749, Republic of Korea. E-mail: jschung@mail.ulsan.ac.kr; Fax: +82522591689; Tel: +82522592249
bSchool of Chemical and Biological Engineering, Seoul National University, Shinlim-dong, kwanak-ku, Seoul 151-744, Republic of Korea
First published on 9th May 2014
Abstract
We report a simple method to prepare N-doped mesoporous reduced graphene oxide (RGO) via the pyrolysis of poly (methyl methacrylate)–graphene oxide (PMMA–GO) and PMMA–RGO composites in a mixed nitrogen and ammoniac atmosphere. The pyrolysis of the PMMA–GO resulted in a heavily nitrogen-doped RGO aerogel with a nitrogen content of more than 5.0 at%, whereas crumpled RGO with a surface area of up to 766 m2 g−1 was obtained by the pyrolysis of PMMA–RGO. A supercapacitor based on the N-doped mesoporous RGOs exhibited an excellent specific capacitance of up to 290 F g−1 at a current density of 1 A g−1 and good electrochemical stability with a capacitance retention of about 85% after 1000 cycles. Moreover, by using an ionic liquid as an electrolyte within a potential range of 0–4 V, a remarkable energy density of up to 149 W h kg−1 was achieved.
1. Introduction
Supercapacitors, a class of electrochemical energy storage devices with ultrahigh power density, fast charging, and exceptionally long cycling life, have attracted increasing attention due to their widespread application in energy back-up systems, portable devices, power tools, and hybrid electric vehicles.1–4 Supercapacitors can be classified into two categories based on the charge-storage mechanism: electrical double-layer capacitors (EDLCs), where the capacitance arises from charge separation at an electrode/electrolyte interface, and pseudocapacitors, where the capacitance comes from Faradaic reactions at the electrode/electrolyte surface. For EDLCs, specific surface area and electrical conductivity of the electrode are crucial to ensure good performance.3,4 Graphene, a one-atom-thick 2D single layer of sp2-bonded carbon, has been considered as an ideal supercapacitor electrode material due to its extremely large surface area, extraordinarily high electrical conductivity, good chemical stability, and high mechanical strength.3–5 However, graphene sheets have a tendency to restack themselves during the synthesis and electrode preparation procedures, resulting in a loss of effective surface area.6–8 Several approaches have been developed to prevent the restacking of graphene during processing, including the fabrication of highly corrugated and crumpled graphene sheets,6–8 the use of guest materials as spacers,9–11 and template-directed or controlled assembly of graphene sheets into three-dimensional (3D) porous structures.12–16 Among the aforementioned processing strategies, the template-directed or controlled assembly method produces 3D graphene-based frameworks such as aerogels, foams, and sponges that exhibit a continuously interconnected macroporous structure, low mass density, large surface area, and high electrical conductivity. The interconnected macropores serve as both ion-buffering reservoirs and low-resistant channels for ion diffusion, which are promising characteristics for high-rate supercapacitor electrodes.14In the template-directed assembly of graphene, polymer latex particles are usually used as a hard template because they are easily removed by pyrolysis14,15 or solvent etching.16 Chen et al.14 prepared 3D macroporous “bubble” graphene films by thermally annealing a vacuum-assisted self-assembly of graphene oxide (GO) and poly(methyl methacrylate) (PMMA) latex particles. The resulting films were then evaluated as supercapacitor electrodes. When compared to a compact graphene film, an extraordinarily high electrochemical capacitance with high rate capacity was observed. In another study, Choi et al.16 used polystyrene microspheres as a template to assemble reduced graphene oxide (RGO) sheets into a 3D macroporous graphene framework. Specifically, a colloidal suspension of RGO and polystyrene latex was filtered and the polystyrene template was subsequently removed via solvent etching. In both of the above-cited studies, the surface area of the 3D macroporous graphene films increased significantly, up to 128.2 m2 g−1 for the macroporous “bubble” graphene film and 194.2 m2 g−1 for the macroporous graphene framework. While such surface areas are about 10 times higher than those of compact graphene films, they are still much smaller than that of single-layered graphene (2630 m2 g−1),3 indicating significant stacking of the graphene sheets forming the wall. To increase the surface area of the graphene framework, the thickness of the framework wall must be decreased. However, the wall must be sufficiently thick so as to prevent collapse of the framework. The use of polymer microspheres results in large pores that require thick walls to maintain the graphene framework and vice versa. In our previous work, we reported that highly conductive PMMA–RGO composite prepared by self-assembly of PMMA latex and GO through electrostatic interaction using PMMA nanospheres with the size of 200 nm.17
It is well-known that the incorporation of nitrogen group into the basal plane or edge of graphene sheets greatly improved the capacitance of graphene electrode.18–22 Jeong et al. reported that the nitrogen-doped graphene prepared by simple plasma process exhibited capacitance 4 times higher than that of pristine graphene.18 Zhang et al. also found that the capacitance of activated microwave expanded graphite oxide (aMEGO) increased from 160 F g−1 for pristine aMEGO to 440 F g−1 for 2.3 at% nitrogen doped aMEGO.19 The great improvement of capacitance of nitrogen doped graphene was attributed to the contribution of pseudocapacitance of nitrogen functional group as well as improving electrode/electrolyte wettability.18,19,22
In this study, we demonstrate that the pyrolysis of nanosphere PMMA–GO results in high surface area of a graphene framework, up to 627 m2 g−1. More interesting, the pyrolysis of PMMA–GO in a mixed nitrogen and ammoniac atmosphere resulted in highly nitrogen-doped mesoporous RGO with a nitrogen content of more than 5.0 at%. The combination of a 3D structure, large surface area, and high nitrogen-doping makes mesoporous RGO a promising material for supercapacitor applications. In a two-electrode symmetric supercapacitor test using ionic liquid as an electrolyte, excellent performance with a specific capacitance of more than 250 F g−1 at a current density of 2 A g−1 was observed, leading to energy and power densities of approximately 150 W h kg−1 and 1900 W kg−1, respectively. Moreover, the nitrogen-doped mesoporous RGO exhibits good cycling stability with a retention of 95% after 1000 cycles.
2. Experimental
2.1. Materials
Expandable graphite (Grade 1721) was provided by Asbury Carbon. Concentrated sulfuric acid (H2SO4), potassium permanganate (KMnO4), hydrochloric acid (HCl), hydrogen peroxide (H2O2), and ammoniac solution were purchased from Samchun Chemicals (Korea). Methyl methacrylate (MMA), 2,2′-azobis (2-methylpropionamidine) dihydrochloride, and 1-ethyl-3-methylimidazolium tetrafluoroborate (EMIMBF4) were purchased from Sigma Aldrich. All chemicals were used as received without further purification.2.2. Preparation of nitrogen-doped mesoporous RGOs
Graphene oxide was prepared by the modified Hummers method using expanded graphite as a starting material (see details in the ESI†).23 The as-synthesized graphene oxide (10 mg mL−1) was diluted with deionized water to 1.0 mg mL−1 and sonicated in an ultrasonic bath (Jeiotech UC-10, 200 W) for 10 min to produce a homogeneous graphene oxide dispersion. Positively charged PMMA latex was synthesized by surfactant-free emulsion polymerization using a cationic free radical initiator, as described in a previous report (see details in the ESI†).17Nitrogen-doped mesoporous RGOs were prepared by the self-assembly of negatively charged GO and positively charged PMMA via electrostatic interactions, followed by a thermal annealing treatment at 500 °C in a mixed nitrogen and ammoniac atmosphere. In a typical procedure, 200 g of PMMA latex (10 wt%) was gradually added to 1.0 L of the GO dispersion (1.0 mg mL−1, PMMA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GO wt/wt ratio 100
GO wt/wt ratio 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) under vigorous stirring for 30 min. The suspension of PMMA–GO was then divided into two parts. The first part was centrifuged at 5000 rpm for 1 h. After decanting the supernatant, the PMMA–GO was dried at 80 °C for 12 h. The second part was reduced with hydrazine (GO–hydrazine 1
5) under vigorous stirring for 30 min. The suspension of PMMA–GO was then divided into two parts. The first part was centrifuged at 5000 rpm for 1 h. After decanting the supernatant, the PMMA–GO was dried at 80 °C for 12 h. The second part was reduced with hydrazine (GO–hydrazine 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 w/w) at 80 °C for 3 h. The obtained colloidal suspension of PMMA–RGO composite was filtered, washed with methanol 3 times, and then dried at 80 °C for 12 h. Finally, the PMMA–GO and PMMA–RGO were thermally annealed at 500 °C in a tube furnace for 3 h under a mixed nitrogen and ammoniac atmosphere at a flow rate of 5000 mL min−1. The mixed nitrogen and ammoniac atmosphere was generated by bubbling nitrogen gas through a concentrated ammoniac solution. The products obtained from the thermal annealing of PMMA–GO and PMMA–RGO are denoted as TRGO and CRGO, respectively.
10 w/w) at 80 °C for 3 h. The obtained colloidal suspension of PMMA–RGO composite was filtered, washed with methanol 3 times, and then dried at 80 °C for 12 h. Finally, the PMMA–GO and PMMA–RGO were thermally annealed at 500 °C in a tube furnace for 3 h under a mixed nitrogen and ammoniac atmosphere at a flow rate of 5000 mL min−1. The mixed nitrogen and ammoniac atmosphere was generated by bubbling nitrogen gas through a concentrated ammoniac solution. The products obtained from the thermal annealing of PMMA–GO and PMMA–RGO are denoted as TRGO and CRGO, respectively.
2.3. Characterization
The morphology of the RGOs was examined by scanning electron microscopy (SEM, JEOL JSM-6500 FE). The porous structure of RGOs was characterized by transmission electron microscopy (TEM, JEOL JEM-1400). X-ray photoelectron spectroscopy (XPS) analysis was performed using a K-alpha spectrometer (Thermal Scientific) with monochromatic Al Kα radiation (hν = 1486.6 eV). Raman spectra were measured with a confocal Raman microscope (Thermo Scientific) utilizing incident laser light with a wavelength of 532 nm. N2 sorption measurements were conducted with a Micromeritics ASAP 2020 instrument. The specific surface area was calculated using the Brunauer–Emmett–Teller (BET) method. Electrochemical measurements were performed on a VSP electrochemical workstation (BioLogic).2.4. Preparation of electrodes and electrochemical measurements
The RGO electrodes were prepared by grinding the RGOs into fine powders, mixing them with polytetrafluoroethylene in a mass ratio of 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15, and then rolling the product into sheets of uniform thickness (∼0.3 mm for CRGO and ∼1.0 mm for TRGO). The RGO films were subsequently punched into electrodes with a diameter of 0.9 mm and dried at 100 °C for 12 h in a vacuum oven. The mass loading of the active material was about 2.5–3.0 mg. Prior to electrochemical testing, the electrodes were soaked in a EMIMBF4 electrolyte for 24 h.
15, and then rolling the product into sheets of uniform thickness (∼0.3 mm for CRGO and ∼1.0 mm for TRGO). The RGO films were subsequently punched into electrodes with a diameter of 0.9 mm and dried at 100 °C for 12 h in a vacuum oven. The mass loading of the active material was about 2.5–3.0 mg. Prior to electrochemical testing, the electrodes were soaked in a EMIMBF4 electrolyte for 24 h.
The supercapacitive performance of the RGOs was characterized using a Teflon Swagelok-type two-electrode configuration with nickel foam as the current collector, a glass fiber membrane as a separator, and ionic liquid EMIMBF4 as an electrolyte. Cyclic voltammetry (CV) and galvanostatic charge–discharge tests were carried out in a potential range of 0–4.0 V. Electrochemical impedance spectroscopy tests were performed over a frequency range of 100 kHz to 0.01 Hz at an open circuit potential with an ac perturbation of 5.0 mV. The mass specific capacitance, energy density, and power density were calculated according to the following equations:13
| C = 2(IΔt/mΔV) |
| E = (1/8)C(ΔV)2 |
| P = E/Δt |
3. Results and discussion
3.1. Nitrogen-doped mesoporous RGOs
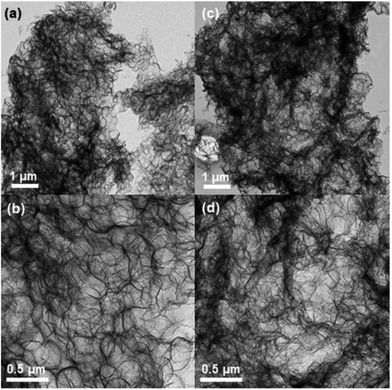
The procedure for fabricating the nitrogen-doped mesoporous RGOs is illustrated in Fig. 1. The self-assembly of PMMA and GO occurred quite easily upon mixing due to electrostatic interactions between the positively charged PMMA particles and negatively charged GO sheets.17 PMMA particles played the role of a hard template, preventing the restacking of GO and RGO sheets. As shown in Fig. S1,† GO and RGO sheets were tightly wrapped the PMMA particles. The morphology of PMMA–GO was compact whereas that of PMMA–RGO was loose. The difference in morphology of PMMA–GO and PMMA–RGO can be attributed to difference in surface properties of GO and RGO in combination with the synthesis condition. The loose morphology of PMMA–RGO can be attributed to the decrease of negative charge on the surface of RGO sheets due to removal of functional groups during hydrazine reduction, resulted in less interaction between RGO sheets and PMMA particles. Moreover, during the hydrazine reduction, RGO became hydrophobic and aggregated with each other. However, in the stirring condition during the hydrazine reduction, the aggregation of RGO sheets was occurred in small scale to produce microscale aggregated PMMA–RGO particles.Nitrogen-doped mesoporous TRGO and CRGO were produced by the thermal annealing of PMMA–GO and PMMA–RGO in a mixed nitrogen and ammoniac atmosphere. During the annealing procedure, the PMMA template was removed via pyrolysis and either GO was reduced or RGO was reduced further. It is interesting to note that the size and shape of the input PMMA–GO was almost preserved in the TRGO, as displayed in Fig. 2a. An SEM image of the broken surface in Fig. 2c shows that the TRGO has a three-dimensional porous structure with a pore size of about 200–300 nm. In contrast, shrinkage and collapse occurred in the case of CRGO, resulting in large agglomerated particles (Fig. 2d). The volume of the CRGO was only about 20% of the input PMMA–RGO volume. Furthermore, the SEM images in Fig. 2e and f reveal that the CRGO sheets were highly crumpled, wrinkled, and folded. TEM was further used to characterize the porous structure of TRGO and CRGO. As shown in Fig. 3(a) and (b), TEM images of TRGO revealed highly porous structure consisting of thin interconnected TRGO walls. It can clearly be seen that the spherical macropores with the size of 200–250 nm were created by pyrolysis of PMMA particles template. For CRGO, TEM images also showed a porous structure. However, CRGO walls were denser and crumpled which is consistent with the SEM images.
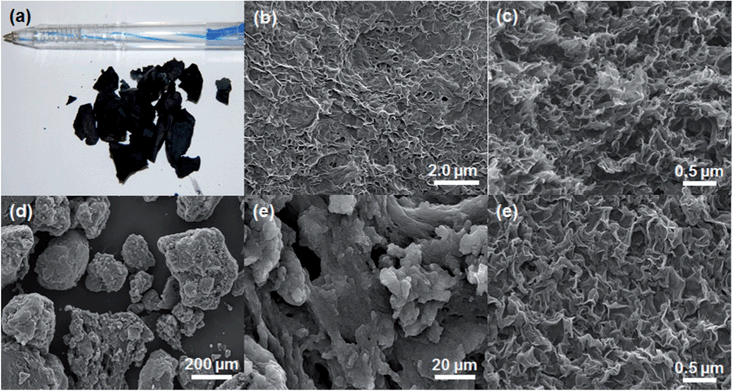 | ||
| Fig. 2 (a) Photograph and (b) SEM image of the skin surface. (c) SEM image of the broken surface of TRGO and (d–f) SEM images of CRGO. | ||
The aerogel porous structure of the TRGO and highly crumpled/wrinkled morphology of the CRGO should provide a high surface area. The N2 adsorption–desorption isotherms of the TRGO and CRGO, shown in Fig. 4a, are characteristic of type IV with very large H3-type hysteresis loops, indicating a mesoporous structure with slit-like pores.15 The pore size distributions of the TRGO and CRGO in Fig. 4b show that the average pore size for both structures is approximately 3.0 nm. The BET specific surface areas of the TRGO and CRGO, as determined from the N2 adsorption–desorption isotherms, are 627 and 766 m2 g−1, respectively, which are 3–5 times higher than that of porous RGOs previously synthesized using a polymer template.14,15 Moreover, these surface areas are significantly higher than many curved, crumpled, corrugated, and aerogel RGOs (Table 1).6–8,13,19
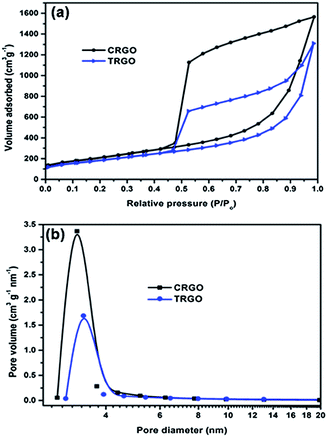 | ||
| Fig. 4 (a) Nitrogen adsorption–desorption isotherms and (b) pore size distribution of TRGO and CRGO. | ||
| RGO | Morphology | BET surface area (m2 g−1) | Pore volume (cm3 g−1) | Ref. |
|---|---|---|---|---|
| Curved RGO | Curved graphene sheet | 500.6 | — | 6 |
| HCGS | Highly corrugated graphene sheet | 517.9 | 1.45 | 7 |
| Crumpled graphene ball | Crumpled, ball-like graphene particles | 567 | — | 8 |
| GH-Hz8 | Graphene hydrogel | 215 | — | 13 |
| NG | Nitrogen-doped graphene | 630.6 | — | 20 |
| C-NGNSs | Crumpled nitrogen doped graphene | 465 | 3.42 | 21 |
| MGF | 3D macroporous bubble graphene film | 128 | — | 14 |
| Ps-in-RGO | 3D porous RGO | 201 | — | 15 |
| TRGO | 3D porous RGO | 627 | 2.1 | This work |
| CRGO | Crumpled RGO | 766 | 2.4 | This work |
It is interesting to note that the surface area and pore volume of the crumpled CRGO are significantly higher than those of the aerogel TRGO. An attempt to increase the TRGO surface area by decreasing the mass ratio of PMMA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GO failed due to collapse of the TRGO structure during annealing. However, it was possible to slightly increase the surface area of the CRGO from 766 to 782 m2 g−1 by decreasing the PMMA
GO failed due to collapse of the TRGO structure during annealing. However, it was possible to slightly increase the surface area of the CRGO from 766 to 782 m2 g−1 by decreasing the PMMA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GO mass ratio from 100
GO mass ratio from 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 to 100
5 to 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, respectively. However, further decreases in the PMMA
3, respectively. However, further decreases in the PMMA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GO mass ratio resulted in a slight decrease of the CRGO surface area.
GO mass ratio resulted in a slight decrease of the CRGO surface area.
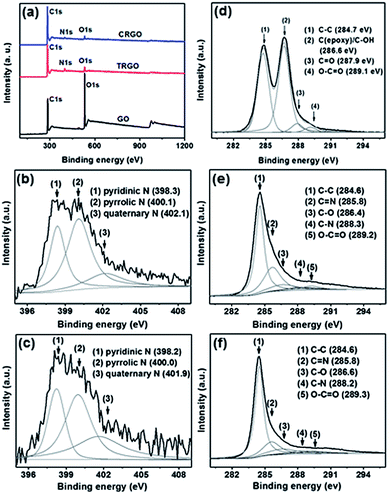 | ||
| Fig. 5 (a) XPS survey spectra, (b and c) N 1s spectra of TRGO and CRGO, and (d–f) C 1s spectra of GO, TRGO, and CRGO. | ||
Thermal annealing of the GO or RGO in an ammoniac atmosphere is a simple and efficient method to dope RGO with nitrogen.20,22,24–26 The efficiency of thermal reduction for GO and RGO, i.e., the efficiency of nitrogen doping upon thermal annealing of the PMMA–GO and PMMA–RGO in a mixed nitrogen and ammoniac atmosphere, was characterized by XPS. XPS survey spectra of the GO and RGOs show that the intensity of the O 1s peak of the RGOs decreased significantly, and a new peak assigned to N 1s appeared at about 400.0 eV (Fig. 5a).22 Deconvolution of the C 1s XPS spectra of the RGOs reveals that most of the oxygen functional groups were removed after the thermal annealing treatment. The elemental compositions of the GO and RGOs are given in Table S1.† A large increase in the C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O atomic ratio was observed, from 2.2 for GO to 13.9 and 21.7 for TRGO and CRGO, respectively. The C
O atomic ratio was observed, from 2.2 for GO to 13.9 and 21.7 for TRGO and CRGO, respectively. The C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O ratio of CRGO is much higher than that of TRGO and can be attributed to a combination of hydrazine reduction and thermal reduction. It is well known that hydrazine reduction of GO results in 3.0–5.0 at% nitrogen doping in RGO.27–29 However, the nitrogen content in CRGO is only 2.26 at%, indicating that nitrogen doping of CRGO did not occur during thermal annealing. A control sample was fabricated by the thermal annealing of PMMA–RGO in nitrogen only so as to confirm whether the CRGO was doped with nitrogen during the thermal treatment. The nitrogen content of the control sample was found to be 2.18 at%, which is similar to that of CRGO. Such a finding confirms that nitrogen doping of the CRGO did not occur during thermal annealing. According to Li et al.,24 certain oxygen functional groups in as-synthesized GO, including carbonyl, carboxylic, lactone, and quinine groups, are responsible for reacting with ammoniac to form C–N bonds, thereby allowing for N-doping. When these oxygen functional groups are removed, the reactivity with ammoniac is significantly reduced, resulting in a lower degree of N-doping in graphene. The absence of slight nitrogen doping during thermal annealing is due to the fact that most of the oxygen functional groups have been removed after hydrazine reduction. In contrast to CRGO, TRGO is heavily nitrogen-doped with a nitrogen content of up to 5.17 at%, which is 2-fold higher than that of CRGO. In the deconvoluted N 1s XPS spectra of the RGOs, there are two shape peaks at 398.1 and 401.0 eV, and a broad peak at 402.2 eV, which are assigned to pyridinic N, pyrrolic N, and quaternary N, respectively.22 Pyrrolic N is the prominent composition in both RGOs.
O ratio of CRGO is much higher than that of TRGO and can be attributed to a combination of hydrazine reduction and thermal reduction. It is well known that hydrazine reduction of GO results in 3.0–5.0 at% nitrogen doping in RGO.27–29 However, the nitrogen content in CRGO is only 2.26 at%, indicating that nitrogen doping of CRGO did not occur during thermal annealing. A control sample was fabricated by the thermal annealing of PMMA–RGO in nitrogen only so as to confirm whether the CRGO was doped with nitrogen during the thermal treatment. The nitrogen content of the control sample was found to be 2.18 at%, which is similar to that of CRGO. Such a finding confirms that nitrogen doping of the CRGO did not occur during thermal annealing. According to Li et al.,24 certain oxygen functional groups in as-synthesized GO, including carbonyl, carboxylic, lactone, and quinine groups, are responsible for reacting with ammoniac to form C–N bonds, thereby allowing for N-doping. When these oxygen functional groups are removed, the reactivity with ammoniac is significantly reduced, resulting in a lower degree of N-doping in graphene. The absence of slight nitrogen doping during thermal annealing is due to the fact that most of the oxygen functional groups have been removed after hydrazine reduction. In contrast to CRGO, TRGO is heavily nitrogen-doped with a nitrogen content of up to 5.17 at%, which is 2-fold higher than that of CRGO. In the deconvoluted N 1s XPS spectra of the RGOs, there are two shape peaks at 398.1 and 401.0 eV, and a broad peak at 402.2 eV, which are assigned to pyridinic N, pyrrolic N, and quaternary N, respectively.22 Pyrrolic N is the prominent composition in both RGOs.
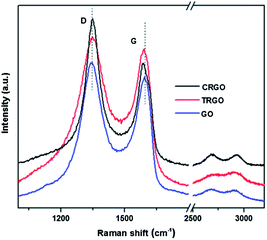
Fig. 6 shows the Raman data obtained for GO and RGO. The spectra were characterized according to two main features: the G-band and D-band. The Raman spectrum of GO exhibited a G-band at 1592 cm−1 and a D band at 1343 cm−1 with a band intensity ratio, I(D)/I(G), of approximately 1.05. After thermal reduction, the G-band of TRGO exhibited a slight blue-shift to 1586 cm−1 and the I(D)/I(G) ratio was almost unchanged. For CRGO, the combination of chemical and thermal reduction resulted in a blue-shift of the G-band to 1582 cm−1 and large increase in the I(D)/I(G) ratio up to 1.44. The larger blue-shift of the G-band and more significant increase of the I(D)/I(G) ratio for CRGO when compared to those of TRGO indicate better recovery of the conjugated C sp2 for CRGO.27
3.2. Electrochemical performance of N-doped mesoporous RGO supercapacitors
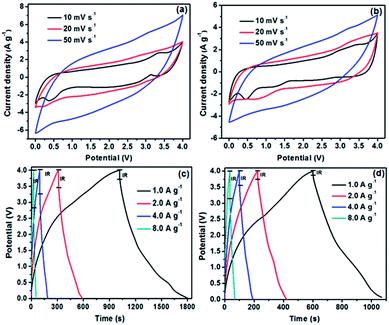
The electrochemical performance of TRGO and CRGO supercapacitor electrodes was evaluated by CV, galvanostatic charge–discharge, and EIS tests in a symmetrical two-electrode system. Fig. 7a and b shows the CVs of TRGO and CRGO supercapacitors in the potential range of 0–4.0 V at different scan rates (10–50 mV s−1). All of the CV curves were quasi-rectangular and slightly oblique, demonstrating the contribution of Faradaic pseudocapacitance. The CV curves obtained at a scan rate of 10 mV s−1 display small redox peaks at about 3.4 V, especially the CV of TRGO, which can be attributed to a high nitrogen dopant concentration.19 To further confirm the contribution of Faradaic pseudocapacitance from N doping, CV tests were performed over a potential range of 0–3.0 V. The obtained CV curves for both TRGO and CRGO were rectangular, suggesting pure electric double-layer capacitive properties (Fig. S3a and c†). However, the areas surrounded by the CV curves in the range of 0–3.0 V were much smaller than those for the CV curves acquired from 0–4.0 V at the same scan rates, indicating a significant contribution from Faradaic pseudocapacitance. The galvanostatic charge–discharge curves of TRGO and CRGO at various current densities were asymmetrically triangular at low current density and isosceles triangular at high current density, implying the electrodes have good capacitive characteristics and electrochemical reversibility (Fig. 7c and d). These observations were consistent with the results of CV measurements. The IR drops in the CV curves of CRGO were significantly smaller than those of TRGO, which indicates lower internal resistance in the electrode. The lower internal resistance of CRGO can be attributed to its good electrical conductivity (high C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O ratio). The specific capacitances calculated from the slope of the discharge curves at different current densities are shown in Fig. 8a. TRGO exhibited excellent specific capacitance, up to 290.3 F g−1 at a current density of 1.0 A g−1, one of the highest specific capacitances ever reported for RGO when using an ionic liquid as an electrolyte (Table 2). The excellent specific capacitance of TRGO can be attributed to the combination of a high surface area, an aerogel structure, and high nitrogen doping. However, as observed with other pseudocapacitive materials,31 the rate performance of TRGO was not remarkable.
O ratio). The specific capacitances calculated from the slope of the discharge curves at different current densities are shown in Fig. 8a. TRGO exhibited excellent specific capacitance, up to 290.3 F g−1 at a current density of 1.0 A g−1, one of the highest specific capacitances ever reported for RGO when using an ionic liquid as an electrolyte (Table 2). The excellent specific capacitance of TRGO can be attributed to the combination of a high surface area, an aerogel structure, and high nitrogen doping. However, as observed with other pseudocapacitive materials,31 the rate performance of TRGO was not remarkable.
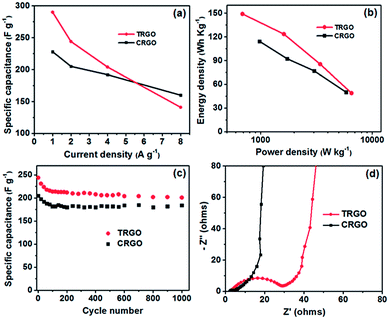 | ||
| Fig. 8 (a) Specific capacitance at different current densities, (b) Ragone plots, (c) cycling stability, and (d) Nyquist plots of TRGO and CRGO. | ||
| RGO | Description | Ionic liquid | Specific capacitance (F g−1) | Energy density (W h kg−1) | Ref. |
|---|---|---|---|---|---|
| Curved RGO | Curved graphene sheet | EMIMBF4 | 154.1 | 85.6 | 6 |
| NG | N-doped RGO | Et4NBF4 | 138.1 | 76.7 | 20 |
| C-NGNSs | Crumpled N-doped RGO | Bu4NBF4 | 245.9 | — | 21 |
| SSG | Solvated RGO | EMIMBF4 | 273.1 | 150.9 | 30 |
| TRGO | 3D porous RGO | EMIMBF4 | 290.3 | 149.1 | This work |
| CRGO | Crumpled RGO | EMIMBF4 | 228.7 | 114.3 | This work |
The specific capacitance of TRGO dropped sharply with an increase in the current density, from 290.3 F g−1 at a current density of 1.0 A g−1 to 141.8 F g−1 at a current density of 8.0 A g−1. Such a decrease corresponds to a capacitance loss of 51.1%. In contrast, CRGO has a lower specific capacitance, only 228 F g−1 at a current density of 1.0 A g−1, but its rate performance was much better, with only a 29.8% capacitance loss over the same current density range. The good rate performance of CRGO can be explained by less contribution of the Faradaic pseudocapacitance due to low level of nitrogen doping in the structure.
In various applications, low energy density is the major challenge for supercapacitors when compared to batteries. Graphene-based supercapacitors using an ionic liquid electrolyte were recently reported to have remarkable energy density characteristics (Table 2). Both TRGO and CRGO exhibited excellent energy densities with maximum values of up to 149.1 and 114.3 W h kg−1, respectively (Fig. 8b). These values are comparable to those obtained with various commercial batteries.6,30
Cycling stability, one of the most important properties of supercapacitor electrodes, was evaluated using the galvanostatic charge–discharge technique at a current density of 2.0 A g−1. As shown in Fig. 8c, the specific capacitances of both TRGO and CRGO gradually decreased over the initial 100–120 cycles, then became stable. The capacitance retentions of TRGO and CRGO after 1000 charge–discharge cycles were 83.6 and 89.2%, respectively, indicating that the electrodes have good long-term electrochemical stability.
Electrochemical impedance spectroscopy was also used to characterize the electrochemical behavior of the TRGO and CRGO electrodes. The Nyquist plots in Fig. 8d exhibit a typical arc in the high-frequency region and a straight line in the low-frequency region. The vertical shape of the straight lines in the low-frequency region indicates that both CRGO and TRGO closely resemble an ideal capacitor.5,6 Furthermore, the high-frequency loop is related to the electronic resistance. The intersection of the curves at the X-axis represents the internal resistance or equivalent series resistance (ESR) of the electrodes associated with the porous structure of the material, which determines the rate at which the supercapacitor can be charged/discharged (i.e., the power capability).5 It can clearly be seen that the ESR of the CRGO electrode is much smaller than that of the TRGO electrode, indicating efficient access of the electrolyte ions to the CRGO surface and shortening of the ion diffusion path. The low ESR of CRGO could be explained by its combination of high electrical conductivity (very high C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O ratio), large surface area, and large pore size volume.
O ratio), large surface area, and large pore size volume.
4. Conclusions
In this work, N-doped mesoporous RGOs with a high surface area were successfully synthesized by the pyrolysis of PMMA–GO and PMMA–RGO composites. The composites were first prepared by the self-assembly of positively charged PMMA latex and negatively charged GO through electrostatic interactions, followed by hydrazine reduction. The pyrolysis of the PMMA–GO in mixed nitrogen and ammoniac atmosphere resulted in heavily nitrogen-doped aerogel TRGO with a nitrogen content of up to 5.17 at%, whereas crumpled CRGO with a surface area of up to 766 m2 g−1 was obtained by the pyrolysis of PMMA–RGO. Both TRGO and CRGO exhibited excellent specific capacitances, up to 290.3 and 228.7 F g−1, respectively, at a current density of 1 A g−1. The large specific capacitance of TRGO and CRGO can be attributed to a combination of a high specific surface area and nitrogen doping. Using an ionic liquid as an electrolyte over a potential range of 0–4.0 V, remarkable energy densities of up to 149 and 114.3 W h kg−1 were achieved for TRGO and CRGO, respectively. Moreover, TRGO and CRGO exhibit good electrochemical stability with capacitance retention of about 85% after 1000 charge–discharge cycles. Such high-performance supercapacitors with high energy density may be implemented in various applications, including hybrid electric vehicles, plug-in hybrid electric vehicles, and renewable energy systems.Acknowledgements
This work was supported by the 2014 Research Fund of University of Ulsan.Notes and references
- P. Simon and Y. Gogotsy, Nat. Mater., 2008, 7, 845 CrossRef CAS PubMed.
- L. L. Zhang, R. Zhou and X. S. Zhao, J. Mater. Chem., 2010, 20, 5983 RSC.
- Y. Huang, J. Liang and Y. Chen, Small, 2012, 8, 1805 CrossRef CAS PubMed.
- H.-J. Choi, S.-M. Jung, J.-M. Seo, D. W. Chang, L. Dai and J.-B. Baek, Nano Energy, 2012, 1, 534 CrossRef CAS PubMed.
- M. D. Stoller, S. Park, Y. Zhu, J. An and R. S. Ruoff, Nano Lett., 2008, 8, 3498 CrossRef CAS PubMed.
- C. Liu, Z. Yu, D. Neff, A. Zhamu and B. Z. Jang, Nano Lett., 2010, 10, 4863 CrossRef CAS PubMed.
- J. Yan, J. Liu, Z. Fan, T. Wei and L. Zhang, Carbon, 2012, 50, 2179 CrossRef CAS PubMed.
- J. Luo, H. D. Jang and J. Huang, ACS Nano, 2013, 7, 1464 CrossRef CAS PubMed.
- J. Yang, T. Wei, B. Shao, F. Ma, Z. Fan, M. Zhang, C. Zheng, Y. Shang, W. Qian and F. Wei, Carbon, 2010, 48, 1731 CrossRef PubMed.
- Q. Cheng, J. Tang, J. Ma, H. Zhang, N. Shinya and L.-C. Qin, Phys. Chem. Chem. Phys., 2011, 13, 17615 RSC.
- Y. Wang, Y. Wu, Y. Huang, F. Zhang, X. Yang, Y. Ma and Y. Chen, J. Phys. Chem. C, 2011, 115, 23192 CAS.
- Y. Xu, K. Sheng, C. Li and G. Shi, ACS Nano, 2010, 4, 4324 CrossRef CAS PubMed.
- L. Zhang and G. Shi, J. Phys. Chem. C, 2011, 115, 17206 CAS.
- C.-M. Chen, Q. Zhang, C.-H. Huang, X.-C. Zhao, B.-S. Zhang, Q.-Q. Kong, M.-Z. Wang, Y.-G. Yang, R. Cai and D. S. Su, Chem. Commun., 2012, 48, 7149 RSC.
- D. Fan, Y. Liu, J. He, Y. Zhou and Y. Yang, J. Mater. Chem., 2012, 22, 1396 RSC.
- B. G. Choi, M. Yang, W. H. Hong, J. W. Choi and Y. S. Huh, ACS Nano, 2012, 6, 4020 CrossRef CAS PubMed.
- V. H. Pham, T. T. Dang, S. H. Hur, E. J. Kim and J. S. Chung, ACS Appl. Mater. Interfaces, 2012, 4, 2630 CAS.
- H. M. Jeong, J. W. Lee, W. H. Shin, Y. J. Choi, H. J. Shin, J. K. Kang and J. W. Choi, Nano Lett., 2011, 11, 2472 CrossRef CAS PubMed.
- L. L. Zhang, X. Zhao, H. Ji, M. D. Stoller, L. Lai, S. Murali, S. Mcdonnell, B. Cleveger, R. M. Wallace and R. S. Ruoff, Energy Environ. Sci., 2012, 5, 9618 CAS.
- Y. Qiu, X. Zhang and S. Yang, Phys. Chem. Chem. Phys., 2011, 13, 12554 RSC.
- Z. Wen, X. Wang, S. Mao, Z. Bo, H. Kim, S. Cui, G. Lu, X. Feng and J. Chen, Adv. Mater., 2012, 24, 5610 CrossRef CAS PubMed.
- H. Wang, T. Maiyalagan and X. Wang, ACS Catal., 2012, 2, 781 CrossRef CAS.
- V. H. Pham, T. T. Dang, T. V. Cuong, S. H. Hur, B.-S. Kong, E. J. Kim and J. S. Chung, Korean J. Chem. Eng., 2012, 29, 680 CrossRef CAS PubMed.
- X. Li, H. Wang, J. T. Robinson, H. Sanchez, G. Diankov and H. Dai, J. Am. Chem. Soc., 2009, 131, 15939 CrossRef CAS PubMed.
- D. Geng, Y. Chen, Y. Chen, Y. Li, R. Li, X. Sun, S. Ye and S. Knights, Energy Environ. Sci., 2011, 4, 760 CAS.
- T. N. Huan, T. V. Khai, Y. Kang, K. B. Shim and H. Chung, J. Mater. Chem., 2012, 22, 14756 RSC.
- S. Stankovich, D. A. Dikin, R. D. Piner, K. A. Kohlhaas, A. Kleinhammes, Y. Jia, Y. Wu, S. T. Nguyen and R. S. Ruoff, Carbon, 2007, 45, 1558 CrossRef CAS PubMed.
- D. Li, M. B. Müller, S. Gilje, R. B. Kaner and G. G. Wallace, Nat. Nanotechnol., 2008, 3, 101 CrossRef CAS PubMed.
- T. T. Dang, V. H. Pham, S. H. Hur, E. J. Kim, B.-S. Kong and J. S. Chung, J. Colloid Interface Sci., 2012, 376, 91 CrossRef CAS PubMed.
- X. Yang, J. Zhu, L. Qui and D. Li, Adv. Mater., 2011, 23, 2833 CrossRef CAS PubMed.
- G. Wang, L. Zhang and J. Zhang, Chem. Soc. Rev., 2012, 41, 797 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Graphene oxide synthesis, PMMA latex synthesis, SEM images of PMMA–GO and PMMA–RGO, elemental composition of TRGO and CRGO, TGA of PMMA, PMMA–GO and PMMA–RGO, CV of TRGO and CRGO in potential window of 0–3.0 V. See DOI: 10.1039/c4ra03049d |
| This journal is © The Royal Society of Chemistry 2014 |