Role of size of drug delivery carriers for pulmonary and intravenous administration with emphasis on cancer therapeutics and lung-targeted drug delivery
Abstract
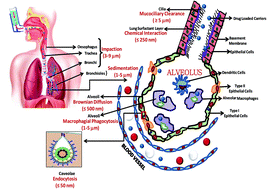
The design of a drug delivery system and the fabrication of efficient, successful, and targeted drug carriers are two separate issues that require slightly different design parameters. The geometry of the drug carrier such as its size and shape, chemical structure, surface chemistry, and surface charge are among the key parameters that need to be optimized to achieve the desired therapeutic behaviour. We review here the effects of the size of the drug delivery carrier on its biodistribution, target specificity, body clearance rate and, most importantly, the therapeutic action of the drug. Pulmonary and intravenous drug administration are the main focus, with special emphasis on cancer therapeutics and lung-targeted drug delivery. The significance of the effect of the dimensional variations and size of the drug delivery carriers on appropriate controlled and targeted drug delivery is explored with the aim of both prohibiting excessive therapeutic loss via different clearance routes and overcoming side-effects and toxicity.

 Please wait while we load your content...
Please wait while we load your content...