Recent advances in H2PO4− fluorescent sensors
Abstract
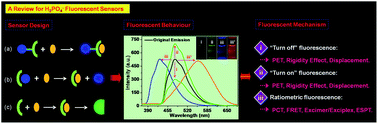
Dihydrogen phosphate (H2PO4−) plays an essential role in a number of chemical and biological processes. The sensitive and selective detection of H2PO4− is of great interest to many scientific fields, ranging from supramolecular chemistry to life sciences. For the detection of H2PO4−, fluorescent methods have plenty of distinct advantages, for example they are simplistic and allow low levels of determination. Therefore, this review will focus on the current progress in the development of H2PO4− fluorescent sensors based on organic scaffolds, for sensing in both organic and aqueous solutions. Three main types of fluorescent probes will be categorized in this review: (i) intensity-based “turn-off” fluorescent sensors; (ii) intensity-based “turn-on” fluorescent sensors; and (iii) ratiometric fluorescent sensors that involve a ratio of two emission outputs. This review should provide a comprehensive description of this research area to date and be instructive for the design and synthesis of new fluorescent sensors for H2PO4−. In addition, the principles and mechanisms employed in the design of H2PO4− sensors will be thoroughly described.

 Please wait while we load your content...
Please wait while we load your content...