Polymer brush functionalized Janus graphene oxide/chitosan hybrid membranes†
Di Hanab,
Peng Xiaob,
Jincui Gub,
Jing Chenb,
Zhiqi Cai*a,
Jiawei Zhang*b,
Wenqin Wangc and
Tao Chen*b
aSchool of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou 510641, China. E-mail: cezqcai@scut.edu.cn
bDivision of Polymer and Composite Materials, Ningbo Institute of Material Technology and Engineering, Chinese Academy of Science, Ningbo 315201, China. E-mail: tao.chen@nimte.ac.cn; zhangjiawei@nimte.ac.cn
cFaculty of Materials Science and Chemical Engineering, Ningbo University, Ningbo 315211, China
First published on 13th May 2014
Abstract
A robust and simple method is reported to prepare polymer brush functionalized Janus graphene oxide (GO)/chitosan hybrid membranes via the combination of interface self-assembly of GO and chitosan, with subsequent self-initiated photografting and photopolymerization (SIPGP) from both sides of the GO/chitosan composite membrane.
Named after the two faced Roman God Janus, the concept of Janus particles comprising multiple compositions and functionalities was introduced by P. G. de Gennes in his Nobel lecture in 1991.1 The term of Janus has thus been extended to describe materials having different properties at opposite sides, which attempt to mimic the behavior of certain molecules, such as surfactants bearing opposite functionalities, leading to fascinating self-assembled behaviors.2 Over the past few years, Janus materials have attracted tremendous attention due to their fantastic properties and potential applications in many fields.3 Yang et al. have developed a series of facile approaches for fabricating inorganic Janus nanosheets, which can serve as solid surfactants to stabilize the emulsion droplets.4–6 Zhao et al. prepared Janus particles which could be anchored at the air–water interface and act as a flexible barrier for preventing coalescence of water droplets.7 Baraban et al. fabricated Janus particles with magnetic caps which were capable of acquiring directed motion by an external magnetic field.8
Furthermore, chemistry in two dimensions differs significantly from chemistry in three-dimensions, which results in the recent interest in thin free-standing two dimensional (2D) Janus materials, such as nanosheets or nanomembranes. These nano materials even could be transported from one environment to another one without losing their structural integrity, yet providing new opportunities for 2D chemistry. Gölzhäuser et al. presented the selective chemical functionalization of free-standing nanomembrane with amino or thiol chemical functionalities on different sides to achieve a 2D Janus nanomembrane by modifying this nanosheet with different fluorescent dyes, respectively.9 Liu et al. reported the preparation of Janus graphene by asymmetrically functionalizing graphene with halogen and phenyl groups.10 Sharma et al. have reported a methyltrimethoxysilane based route to growing a Janus silica film at the oil–water interface, which shows anisotropic wetting by water on its two surfaces.11 These novel Janus structures provide a platform for studying 2D chemistry and graphene devices with multiple functions. However, despite many desirable applications, fabrication of functional self-supporting 2D Janus nanosystems is still a dream of chemists. A more robust and general approach is still highly required.
With abundant oxygen-containing groups on the basal planes and edges, graphene oxide (GO) has been making a profound impact in many areas of science and technology because of its remarkable physicochemical properties and potential applications.12–14 Enormous effort has been focused onto functionalizing GO nanosheets with chemical groups,15 polymer chains,16,17 nanoparticles and nanoplates.18,19 Chen et al. obtained modified GO with nonvolatile rewritable memory effect by attaching triphenylamine-based polyazomethine to GO surface.20 Maser et al. grafted poly(vinyl alcohol) to GO sheets leading to tough GO based materials.21 Functionalization of GO nanosheets have created unexpected properties for advanced potential applications. As excellent candidate for 2D chemistry, Janus asymmetric functions will bring us more surprises in various applications. However, to the best of our knowledge, asymmetrically covalently attaching polymer chains to both sides of the hybrid GO thin sheets has not been reported.
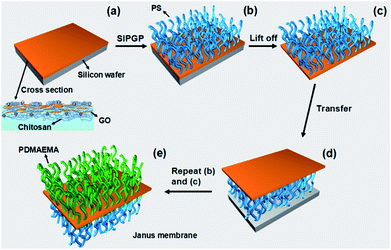
Recently, it was demonstrated that polymer brushes could be prepared even without a surface-bound initiator by self-initiated photografting and photopolymerization (SIPGP).22,23 Herein, we report a robust and facile method to fabricate a polymer brushes grafted Janus graphene oxide/chitosan hybrid membrane which is outlined in Fig. 1. GO/chitosan composite membrane was obtained firstly by interface self-assembly of GO and chitosan induced by electrostatic interaction,24 poly(styrene) (PS) and poly(N,N-dimethylaminoethyl methacrylate) (PDMAEMA) were then grafted from the photoactive sites of upper surface and the lower surface of the GO/chitosan membrane by SIPGP, respectively.
GO nanosheets can be easily synthesized by exfoliating graphite according to a modified Hummers' method,25 and be dispersed uniformly in water for a long time. The thickness of 1.2 ± 0.2 nm and the diameters of 1 to several micrometers of GO nanosheets was determined by atom force microscope (AFM) (Fig. S1†). With negative charge, GO nanosheets behave like negatively charged polyelectrolytes that can interact with positively charged polyelectrolytes to form stable complex. GO/chitosan composite membrane was thus prepared through interface self-assembly of GO with positively charged chitosan solution (Fig. S2†).
There are abundant hydroxyl, carboxyl and amine groups on the membrane, which provides the possibility as photo active site for SIPGP to grow polymer brushes.22,23 By grafting polymers through SIPGP from both sides of GO/chitosan membrane, a polymer brushes functionalized Janus GO/chitosan hybrid membrane was achieved. PS brushes were grafted firstly from the upper side of silicon substrate supported GO/chitosan membrane. After photopolymerization, the membrane was rigorously rinsed with toluene to remove physisorbed polymer and monomer. The PS grafted GO/chitosan was then etched using KOH solution (0.1 g mL−1) at 80 °C to achieve a free-standing membrane (Fig. 1a–c). After a reversal transfer (Fig. 1d), PDMAEMA brushes were subsequently grafted from another side (Fig. 1e). The free-standing polymer brushes grafted Janus GO/chitosan hybrid membrane was finally achieved after the same etching process (Fig. 2a). The membrane is strong enough to be manipulated with tweezers and transferred to silicon wafer. Through asymmetrically bifacial cografting of hydrophilic PDMAEMA and hydrophobic PS on the opposite sides, Janus membrane is supposed to exhibit anisotropic surface wettability. Water contact angle (CA) test was performed to investigate surface CA evolution during the fabrication of polymer brushes grafted Janus GO/chitosan membrane. Once being grafted with PS brushes, the CA was changed from original 60° (upper side of GO/chitosan membrane) to ∼87° (Fig. 2b). Grafting PDMAEMA brushes from the lower surface leads to water CA decreasing from ∼65° (lower side of GO/chitosan membrane) to ∼17° (Fig. 2b). To exhibit Janus membrane's anisotropic surface wettability more directly, two drops of water were applied to two sides of the Janus membrane respectively. The difference of CA reaches as high as ∼70° which demonstrates dramatic disparity on surface properties of two sides (Fig. 2b).
 | ||
| Fig. 2 (a) Optical photographs of free-standing polymer brushes functionalized Janus GO/chitosan hybrid membrane and (b) two drops of water on opposite sides of the Janus hybrid membrane. | ||
In order to investigate the surface morphology evolution during the fabrication of polymer brushes functionalized Janus GO/chitosan hybrid membrane, atom force microscope (AFM) and scanning electronic microscope (SEM) were used. AFM images show that many irregular wrinkles exist on surfaces of the GO/chitosan membrane (Fig. S3†). Consistent with the results of AFM, SEM image show that many wrinkles on upper surface are highly crumpled to form fiber-like structure (Fig. S4†), and crumpled wrinkles are also observed on the lower surface (Fig. S5†). Some distinct raised structures are observed on both sides of GO/chitosan membrane. On the upper surface, the raised structures are isolated to each other with average size of dozens of nanometers (Fig. 3a). Hair-like structures are covered on the lower surface (Fig. 3b). The size and shape of these raised structures can be tuned by varying the concentration of chitosan solution. After modification, the highly crumpled wrinkles on upper surface are not so obvious and island-like structures disappear (Fig. 3c). Compared with unmodified state, the lower surface becomes relatively smooth. The wrinkles and hair-like structures on it are not observed after PDMAEMA brushes are grafted (Fig. 3d).
The membrane turned black from brown after polymer grafting,26 which can be ascribed to partial reduction of GO sheets by UV. Raman spectroscopy was performed to investigate GO reduction. As shown in Fig. 4, Raman spectra of original GO/chitosan membrane and polymer brushes grafted Janus membrane exhibit prominent D and G bands at 1345 cm−1 and 1600 cm−1, respectively. After modification of polymer brushes by SIPGP, the ID/IG ratio increases from 0.93 to 0.99, indicating GO was partially reduced.
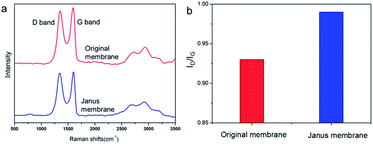 | ||
| Fig. 4 (a) Raman spectra of original membrane and polymer brushes grafted Janus hybrid membrane. (b) ID/IG ratio of original membrane and polymer brushes grafted Janus hybrid membrane. | ||
In order to further understand the structure of polymer brushes grafted Janus GO/chitosan hybrid membrane, cross-sectional SEM was applied to obtain the cross profile of the hybrid membrane. As shown in Fig. 5a, a distinct three-layer “sandwich” structure can be observed by SEM. The upper layer is homogeneous and has an obvious boundary with the middle layer. Its thickness is about 350 nm, which is consistent with thickness increase after photopolymerization. The middle layer is mainly composed of GO/chitosan sheets, and they are stacked in a layered fashion whose surfaces are rough due to encapsulation of chitosan. The bottom layer has a homogeneous structure just like the upper layer which has a thickness of 250 nm. X-ray photoelectron spectroscopy (XPS) was used to verify the Janus structure of the membrane. The maximum of detect depth for XPS is about 10 nm, and the thickness of the grafted layers are 200–400 nm. Therefore, the composition of the two layers can be characterized with XPS without interference. The XPS spectra of both surfaces of Janus membrane are shown in Fig. 5b. XPS survey spectrum of PDMAEMA grafted surface reveals N1s peak at 398 eV, which can be assigned to the tertiary amine group in PDMAEMA. The O1s peak and Si peaks in the XPS spectra are attributed to contamination of SiO2 during etching process. N is the characteristic element to distinguish PS and PDMAEMA, so the two sides can be determined by content of N. C1s spectra for both surfaces of Janus membrane are shown in Fig. 5c and d. The C1s spectrum of PDMAEMA grafted surface is deconvoluted into five component peaks.27 The intensity ratios of these deconvoluted peaks are in good agreement with the stoichiometric ratio of the corresponding carbon atoms in chemical structure of PDMAEMA as [A]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [B]
[B]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [C]
[C]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [D]
[D]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [E] = 2
[E] = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The C1s spectrum of PS grafted surface includes a main hydrocarbon peak at binding energy of 284.8 eV and π–π* shake-up satellites at 391.5 eV.28 These results strongly confirm that PS and PDMAEMA were successfully grafted from the upper and lower surfaces of GO/chitosan membrane, respectively.
1. The C1s spectrum of PS grafted surface includes a main hydrocarbon peak at binding energy of 284.8 eV and π–π* shake-up satellites at 391.5 eV.28 These results strongly confirm that PS and PDMAEMA were successfully grafted from the upper and lower surfaces of GO/chitosan membrane, respectively.
Conclusions
We represent a robust and simple approach to fabricate free-standing polymer brushes functionalized Janus GO/chitosan hybrid membrane. GO/chitosan composite are constructed firstly through interface self-assembly induced by electrostatic interaction. PS and PDMAEMA brushes are respectively grafted from photoactive sites of the upper and lower surfaces of the composite membrane through SIPGP to obtain a distinct Janus structure. SIPGP is a facile polymerization method in which surface-bound initiator is not required, and many vinyl monomers can be grafted from photo active site on the surface of GO/chitosan membrane by SIPGP. Therefore, the compositions of Janus membrane surfaces are alternative in a wide range and the surface properties of GO/chitosan can be tuned in a definite direction by selecting appropriate vinyl monomers. Moreover, the thickness of grafted layer can be modulated by adjusting the irradiation time.29 The new asymmetric polymer brushes functionalized Janus GO/chitosan hybrid membrane opens the way to many potential applications, including separation, sensing and as the versatile material platform for the hybrid with metallic nanoparticles for catalysis applications.Acknowledgements
We thank Chinese Academy of Science for Hundred Talents Program, Chinese Central Government for Thousand Young Talents Program and the National Natural Science Foundation of China (51303195, 21304105), and Excellent Youth Foundation of Zhejiang Province of China (LR14B040001).Notes and references
- P. G. de Gennes, Angew. Chem., Int. Ed., 1992, 31, 842–845 CrossRef.
- M. Lattuada and T. A. Hatton, Nano Today, 2011, 6, 286–308 CrossRef CAS PubMed.
- J. Hu, S. Zhou, Y. Sun, X. Fang and L. Wu, Chem. Soc. Rev., 2012, 41, 4356–4378 RSC.
- F. Liang, K. Shen, X. Qu, C. Zhang, Q. Wang, J. Li, J. Liu and Z. Yang, Angew. Chem., Int. Ed., 2011, 50, 2379–2382 CrossRef CAS PubMed.
- H. Yang, F. Liang, X. Wang, Y. Chen, C. Zhang, Q. Wang, X. Qu, J. Li, D. Wu and Z. Yang, Macromolecules, 2013, 46, 2754–2759 CrossRef CAS.
- Y. Chen, F. Liang, H. Yang, C. Zhang, Q. Wang, X. Qu, J. Li, Y. Cai, D. Qiu and Z. Yang, Macromolecules, 2012, 45, 1460–1467 CrossRef CAS.
- Y. J. Zhao, H. C. Gu, Z. Y. Xie, H. C. Shum, B. P. Wang and Z. Z. Gu, J. Am. Chem. Soc., 2013, 135, 54–57 CrossRef CAS PubMed.
- L. Baraban, D. Makarov, R. Streubel, I. Mönch, D. Grimm, S. Sanchez and O. G. Schmidt, ACS Nano, 2012, 6, 3383–3389 CrossRef CAS PubMed.
- Z. Zheng, C. T. Nottbohm, A. Turchanin, H. Muzik, A. Beyer, M. Heilemann, M. Sauer and A. Gölzhäuser, Angew. Chem., Int. Ed., 2010, 49, 8493–8497 CrossRef CAS PubMed.
- L. Zhang, J. Yu, M. Yang, Q. Xie, H. Peng and Z. Liu, Nat. Commun., 2013, 4, 1443–1449 CrossRef PubMed.
- M. Kulkarni, R. Bandyopadhyaya and A. Sharma, J. Mater. Chem., 2008, 18, 1021–1028 RSC.
- D. Chen, H. B. Feng and J. H. Li, Chem. Rev., 2012, 112, 6027–6053 CrossRef CAS PubMed.
- G. Eda and M. Chhowalla, Adv. Mater., 2010, 22, 2392–2415 CrossRef CAS PubMed.
- K. P. Loh, Q. L. Bao, G. Eda and M. Chhowalla, Nat. Mater., 2010, 2, 1015–1024 CAS.
- S. K. Singh, M. K. Singh, P. P. Kulkarni, V. K. Sonkar, J. J. A. Gracio and D. Dash, ACS Nano, 2012, 6, 2731–2740 CrossRef CAS PubMed.
- H. He and C. Gao, Chem. Mater., 2010, 22, 5054–5064 CrossRef CAS.
- M. Steenackers, A. M. Gigler, N. Zhang, F. Deubel, M. Seifert, L. H. Hess, C. H. Y. X. Lim, K. P. Loh, J. A. Garrido and R. Jordan, J. Am. Chem. Soc., 2011, 133, 10490–10498 CrossRef CAS PubMed.
- J. L. Gunjakar, I. Y. Kim, J. M. Lee, N. S. Lee and S. J. Hwang, Energy Environ. Sci., 2013, 6, 1008–1017 CAS.
- E. Ou, X. Zhang, Z. Chen, Y. Zhan, Y. Du, G. Zhang, Y. Xiang, Y. Xiong and W. Xu, Chem.–Eur. J., 2011, 17, 8789–8793 CrossRef CAS PubMed.
- X. D. Zhuang, Y. Chen, G. Liu, P. P. Li, C. X. Zhu, E. T. Kang, K. G. Neoh, B. Zhang, J. H. Zhu and Y. X. Li, Adv. Mater., 2010, 22, 1731–1735 CrossRef CAS PubMed.
- M. Cano, U. Khan, T. Sainsbury, A. O'Neill, Z. Wang, I. McGovern, W. K. Maser, A. M. Benito and J. N. Coleman, Carbon, 2013, 52, 363–371 CrossRef CAS PubMed.
- M. Steenackers, A. Küller, S. Stoycheva, M. Grunze and R. Jordan, Langmuir, 2009, 25, 2225–2231 CrossRef CAS PubMed.
- P. Xiao, J. C. Gu, J. Chen, D. Han, J. W. Zhang, H. T. Cao, R. B. Xing, Y. C. Han, W. Q. Wang and T. Chen, Chem. Commun., 2013, 49, 11167–11169 RSC.
- J. Zou and F. Kim, ACS Nano, 2012, 6, 10606–10613 CrossRef CAS PubMed.
- X. Zhou and Z. Liu, Chem. Commun., 2010, 46, 2611–2613 RSC.
- S. Park, D. A. Dikin, S. T. Nguyen and R. S. Ruoff, J. Phys. Chem. C, 2009, 113, 15801–15804 CAS.
- S. Gupta, M. Agrawal, M. Conrad, N. A. Hutter, P. Olk, F. Simon, L. M. Eng, M. Stamm and R. Jordan, Adv. Funct. Mater., 2010, 20, 1756–1761 CrossRef CAS.
- C. Ton-That, A. G. Shard, D. O. H. Teare and R. H. Bradley, Polymer, 2001, 42, 1121–1129 CrossRef CAS.
- I. Amin, M. Steenackers, N. Zhang, A. Beyer, X. Zhang, T. Pirzer, T. Hugel, R. Jordan and A. Gölzhäuser, Small, 2010, 6, 1623–1630 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra02826k |
| This journal is © The Royal Society of Chemistry 2014 |