Preparation and electrochemical catalytic application of nanocrystalline cellulose doped poly(3,4-ethylenedioxythiophene) conducting polymer nanocomposites
Jinshi
Fan
a,
Wan
Shao
a,
Guiyun
Xu
b,
Xinyan Tracy
Cui
c and
Xiliang
Luo
*b
aCollege of Chemical Engineering, Qingdao University of Science and Technology, Qingdao 266042, P. R. China
bKey Laboratory of Sensor Analysis of Tumor Marker, Ministry of Education, College of Chemistry and Molecular Engineering, Qingdao University of Science and Technology, Qingdao 266042, P. R. China. E-mail: xiliangluo@hotmail.com
cDepartment of Bioengineering, University of Pittsburgh, Pittsburgh, PA 15260, USA
First published on 23rd May 2014
Abstract
Novel conducting polymer nanocomposites of poly(3,4-ethylenedioxythiophene) (PEDOT) doped with nanocrystalline cellulose (NCC) were prepared through both chemical and electrochemical polymerization methods. The prepared nanocomposites were characterized by scanning electron microscopy, Fourier transform infrared spectroscopy, electrochemical impedance spectroscopy and cyclic voltammetry. In both cases, NCC could be effectively incorporated into the polymerized PEDOT to form uniform conducting polymer nanocomposites that exhibit good electrochemical properties. With NCC acting as the only dopant in the electrochemical polymerization process, pure NCC doped PEDOT (PEDOT/NCC) with a unique nanostructure could be synthesized through the simple electrodeposition method. The electrochemically deposited PEDOT/NCC nanocomposite showed very low electrochemical impedance, large charge storage capacity and good electrocatalytic activity. Based on the excellent catalytic activity of the PEDOT/NCC nanocomposite towards the oxidation of dopamine, a sensitive amperometric sensor for the detection of dopamine with a detection limit of 69 nM was developed.
Introduction
Poly(3,4-ethylenedioxythiophene) (PEDOT) has been considered as one of the most promising conducting polymers, as it can be easily synthesized from the monomer 3,4-ethylenedioxythiophene (EDOT) at low electrochemical potential and the polymer possesses outstanding stability and electrical conductivity.1,2 PEDOT has found broad applications in various fields from solar energy to transistors.3–8 With the advance of nanotechnology, nanocomposites based on PEDOT and different nanomaterials have attracted much attention owing to their unique properties. It was found that properties of PEDOT composites could be remarkably altered when different nanomaterials were used as dopants. For example, electrochemically deposited PEDOT doped with CNTs exhibited high stability and excellent biocompatibility,9 while the nanocomposite of PEDOT and montmorillonite synthesized through in situ polymerization showed excellent electrical conductivity.10 These unique properties, in addition to their capability of being electrochemically synthesized, make PEDOT nanocomposites ideal candidates to be used as electrode materials and for the development of sensors.11–16Nanocrystalline cellulose (NCC), also called cellulose nanocrystals, is typically a nanoscale rod-like crystalline cellulose (with a length of tens to hundreds of nanometers and a diameter of about 1–100 nm) prepared from native cellulose by the method of acid hydrolysis.17,18 NCC possesses several advantages such as low cost, high surface area and nontoxicity.19–21 Moreover, it can form stable lyotropicnematic liquid crystals phase above a critical concentration.22,23 Owing to its specific properties, NCC has received increasing attention in the past years, and various applications of NCC have been investigated. Very recently, surfactant modified NCC and silver nanoparticles have been used to modify poly(lactic acid) nanocomposite for food packaging, and they could significantly increase the barrier effect of the produced composite film.24 Similar application of NCC to reduce the water permeation property of polycaprolactone composite film has also been reported.25 NCC has also been used as a multifunctional cross-linker26 and a biocompatible matrix for enzyme immobilization.27 The application of NCC for the construction of sensors and biosensors has also been explored. For example, a sensitive sensor for the detection of human neutrophil elastase, a biomarker for chronic wounds, has been developed by Edwards and coworkers28 based on peptide conjugated NCC. Shang and coworkers29 have developed an electrical DNA biosensor using carboxylated NCCs attached with Ag nanoparticles as labels. Other recently developed sensing systems based on NCC include colorimetric humidity indicator30 and glucose biosensor.31
The combination of different polymers and NCC may generate potentially promising composites that combine the unique properties of these two components. In the past, a number of research papers about the preparation and application of such composite materials have been reported.32–36 Nanocomposites of polyaniline and NCC prepared in lyotropic chiral nematic liquid crystals have been reported to be electrically conductive with a conductivity up to 0.01 S cm−1.37 Cellulose fibers individually coated with a 50 nm thin layer of polypyrrole formed through chemical polymerization have been used for energy storage, and the obtained nanostructured high surface area electrode material exhibited very high charge capacity and excellent cycling stability.38 These studies are mainly based on polyaniline and polypyrrole, while the preparation and application of nanocomposite of PEDOT and NCC have not yet been explored. In this work, for the first time, we report the preparation of conducting polymer nanocomposites based on PEDOT doped with NCC (PEDOT/NCC) by chemical oxidative polymerization and electrochemical polymerization, respectively. Properties of the prepared PEDOT/NCC nanocomposites were studied in detail, and their electrochemical catalytic activities towards the oxidation of dopamine (DA), was further investigated.
Experimental section
Materials
Cotton pulp in dry lap form was purchased from Weifang Henglian Pulp & Papermaking Co., Ltd (Weifang, Shandong, China). 3,4-Ethylenedioxythiophene (EDOT), lithium perchlorate trihydrate (LiClO4·3H2O) and DA were obtained from Aladdin Reagents (Shanghai, China). All other chemicals were of analytical grade. Deionized water from a Milli-Q water purifying system was used throughout.Apparatus
Electrochemical experiments were performed with a CHI660D electrochemical workstation (Shanghai CH Instruments Co., China) coupled with a conventional three-electrode system. A homemade carbon paste electrode (CPE, prepared according to our previous report39) or modified CPE was used as the working electrode. An Ag/AgCl electrode and a platinum wire were used as the reference and counter electrodes, respectively. Field emission scanning electron microscopy (SEM) was performed with a JEOL JSM-7500F SEM instrument (Hitachi High-Technology Co., Ltd., Japan). The Zeta potential of NCC and PEDOT/NCC was measured with the Malvern Zetasizer Nano ZS90 (Malvern Instruments Ltd, UK). Fourier transform infrared spectroscopy (FTIR) was recorded using the BRUKER TENSOR 70 spectrometer (Bruker Optics, Germany).Preparation of nanocrystalline cellulose
NCC was prepared according to our previous work.17 Briefly, cotton pulp in dry lap form was putted into a Hollander beater until the degree of beating was about 50° SR. 3.0 g of the obtained cotton fibers was put into a three-neck flask, followed by the addition of sulfuric acid (64 wt%, 90 g) and the mixture was stirred for 5 h. The suspension was then filtered with a dialysis bag until the pH of the suspension was 7.0 (at normal atmospheric temperature). Finally, the suspension was dehydrated with organic solvents and dried at 60 °C (until constant weight), and the product NCC was obtained.Chemical polymerization of PEDOT/NCC
10 mL of 1.0 mg mL−1 NCC suspension in deionized water (pH 7.0) was heated to 70 °C under magnetic stirring. Following the addition of 10 mg EDOT to the above suspension, 14 mg FeCl3·6H2O was immediately added, and the suspension was vigorously stirred again for 2 h at the same temperature. After cooling, the resulting material was collected and subjected to a series of centrifugation (3000 rpm for 15 min) and washing cycles until the supernatant became neutral. The obtained material (chemically polymerized PEDOT/NCC nanocomposite) was then dried at 60 °C under vacuum before use.For the preparation of CPE modified with chemically polymerized PEDOT/NCC, 10 μL suspension containing 15 mg mL−1 of the chemically prepared PEDOT/NCC was drop-coated on the CPE and dried under ambient temperature.
Electrochemical polymerization of PEDOT/NCC
The electrochemical preparation of the PEDOT/NCC nanocomposite was performed through electropolymerization in a solution containing 2.0 mg mL−1 NCC and 0.02 M EDOT. The electrochemical polymerization potential was set at 1.2 V, and the polymerization time was 120 s. For comparison, PEDOT conducting polymer without NCC was prepared similarly through the electrochemical polymerization in 0.02 M EDOT solution containing 0.2 M LiClO4.Electrochemical measurements
Electrochemical impedance spectroscopy (EIS) measurements were recorded in 50.0 mM [Fe(CN)6]3−/4− solution containing 0.1 M KCl within a frequency range of 1–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Hz. The amplitude of the applied sine wave was 5 mV, and the direct current potential set at 0.30 V. Cyclic voltammetry (CV) was performed in either phosphate buffered saline (PBS, 0.05 M, pH 7.0) or Britton–Robinson (B–R) buffer (0.2 M, pH 5.0), as indicated in specific experiment conditions. Unless otherwise indicated, the scan rate of CV was 100 mV s−1. DA detection was performed using amperometric current–time (i–t) curve in stirring B–R buffer, with the potential set at 0.34 V. All experiments were conducted at ambient temperature.
000 Hz. The amplitude of the applied sine wave was 5 mV, and the direct current potential set at 0.30 V. Cyclic voltammetry (CV) was performed in either phosphate buffered saline (PBS, 0.05 M, pH 7.0) or Britton–Robinson (B–R) buffer (0.2 M, pH 5.0), as indicated in specific experiment conditions. Unless otherwise indicated, the scan rate of CV was 100 mV s−1. DA detection was performed using amperometric current–time (i–t) curve in stirring B–R buffer, with the potential set at 0.34 V. All experiments were conducted at ambient temperature.
Results and discussion
Preparation and SEM characterization of PEDOT/NCC nanocomposites
As NCC is prepared from cotton pulp by the method of sulfuric acid hydrolysis, it possesses many sulfonic acid ester groups, which makes NCC negatively charged in aqueous solution (the zeta potential of NCC was measured to be −21.9 mV). The negatively charged NCC with quite big specific surface area will be doped and wrapped in the growing polymer to neutralize the positive charges on the backbone of PEDOT (the zeta potential of PEDOT/NCC was measured to be −7.54 mV).SEM images of the chemically polymerized PEDOT/NCC and the electrochemically polymerized PEDOT/NCC both on the CPE surface are shown in Fig. 1. The morphologies of the chemically and electrochemically synthesized PEDOT/NCC nanocomposites are significantly different. The chemically polymerized PEDOT/NCC nanocomposite shows a microstructure composed of packed particles, with the size ranging roughly from 100–140 nm (Fig. 1d, the scale bar is 100 nm). Compared with the chemically polymerized PEDOT without NCC (Fig. 1b), the chemically polymerized PEDOT/NCC exhibits a more regular particle-like microstructure, which should be ascribed to the presence of NCCs that are wrapped by the PEDOT. On the other hand, the electrochemically polymerized PEDOT/NCC nanocomposite (Fig. 1c) exhibits a uniform network-like wrinkled surface morphology, and its microstructure is composed of evenly packed nanorods. Interestingly, the shape of these rods (with the diameter of about 40–60 nm, the length of about 100–150 nm) is similar to that of the starting NCC (Fig. 1a, with the diameter of about 30 nm). This morphology difference between the chemically and electrochemically prepared PEDOT/NCC nanocomposites may be ascribed to their different polymerization mechanisms. For the electrochemical polymerization, NCC is the only dopant for PEDOT, and the polymer prefer to grow around the negatively charged NCC, resulting to the formation of PEDOT coated NCC rods. While for the chemical polymerization using FeCl3 as oxidant, the negative chloride ion can also be incorporated into the formed PEDOT as a dopant. Therefore, the formation of PEDOT is not limited around the NCC surface, and the microstructure of the chemically formed PEDOT/NCC doesn't resemble the morphology of the primitive NCC.
 | ||
| Fig. 1 SEM images of NCC (a), the chemically polymerized PEDOT/chloride (b), the electrochemically polymerized PEDOT/NCC (c), and the chemically polymerized PEDOT/NCC (d). | ||
FTIR characterization of the PEDOT/NCC nanocomposite
FTIR is used to characterize the chemical composition of NCC and the chemically polymerized PEDOT/NCC. The FTIR spectra of NCC (curve a) and the chemically polymerized PEDOT/NCC (curve b) are shown in Fig. 2. As seen in Fig. 2a, the band at around 3348 cm−1 is due to the O–H stretching vibration, the band at about 2902 cm−1 is attributed to the stretching vibration of methylene and methine, and the band at 1429 cm−1 is assigned to the scissoring vibration of methylene. The peaks at 1163 cm−1, 1113 cm−1 and 1059 cm−1 are corresponding to the C–O–C modes in glucose monomer of NCC. All the above characteristic speaks are also seen in Fig. 2b shows that the structure of NCC is still well maintained in the chemically polymerized PEDOT/NCC. The small peak presented at 1530 cm−1 is characteristic of ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) and ν(C–C) vibrations of the thiophene ring,10 while the decreased intensity of the band at around 3348 cm−1 due to the O–H stretching vibration and the more stronger peaks at about 2902 cm−1 due to the stretching vibration of methylene and methine confirms that NCC was combined with the PEDOT, and they affected each other.
C) and ν(C–C) vibrations of the thiophene ring,10 while the decreased intensity of the band at around 3348 cm−1 due to the O–H stretching vibration and the more stronger peaks at about 2902 cm−1 due to the stretching vibration of methylene and methine confirms that NCC was combined with the PEDOT, and they affected each other.
Electrochemical characterization
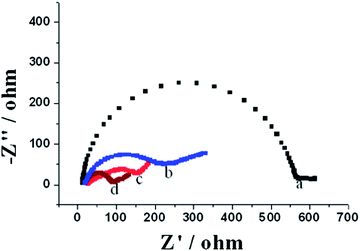
The electrochemical properties of different electrodes are characterized by EIS. Fig. 3 shows the impedance spectra of different modified electrodes in the form of Nyquist plots, which are composed of a semicircle portion corresponding to the charge transfer process and a linear portion attributing to the diffusion limited process at the electrode interface, respectively.40–43 Simply, the diameter of the semicircle in the Nyquist plot is equivalent to the charge transfer resistance (Rct). It is clear that CPEs modified with PEDOT/NCC nanocomposites show much lower Rct than that of the bare CPE, regardless whether the nanocomposites are chemically or electrochemically polymerized. This may be ascribed to the fact that these PEDOT/NCC nanocomposite films are conductive, and they (with their rough and porous microstructures) can increase the effective surface area of the modified electrodes. Interestingly, the Rct of the CPE modified with electrochemically polymerized PEDOT/NCC is lower than that of the CPE modified with chemically polymerized PEDOT/NCC and the CPE modified with electrochemically polymerized PEDOT doped with perchlorate, indicating excellent charge transfer property of the electrochemically polymerized PEDOT/NCC nanocomposite. Therefore, the improved properties are arising from both the electrochemical deposition and NCC.CV was used to evaluate the charge storage capacity of the CPE, PEDOT/NCC modified and PEDOT/chloride modified electrodes using the enclosed area of the CV curve, i.e., the amount of charge passed during one CV cycle.9 It can be seen from Fig. 4, the PEDOT modified CPEs (b and c) exhibits much higher charge storage capacity than the bare CPE (curve a), and the PEDOT doped with NCC shows higher charge storage capacity than that of the PEDOT/perchlorate. Such a high charge storage capacity is associated with the large surface area and good conductivity of the electrochemically polymerized PEDOT/NCC nanocomposite, and it is consistent with the EIS investigation above.
 | ||
| Fig. 4 Cyclic voltammograms of (a) bare CPE, and CPEs modified with electrochemically polymerized PEDOT doped with (b) perchlorate and (c) NCC in pH 7.0 PBS. The scan rate was 100 mV s−1. | ||
The stability of the electrochemically polymerized PEDOT/NCC film was investigated using CV (from −0.6 V to 0.6 V, with a scan rate of 50 mV s−1) electrochemical stimulation for 1500 consecutive oxidation–reduction cycles. It was found that after 1500 CV cycles, the charge storage capacity retention of the PEDOT/NCC modified electrode and the PEDOT/perchlorate modified electrode was about 78% and 75%, respectively, indicating good stability of this PEDOT based nanocomposite.44–46
Electrochemical oxidation of DA at nanocomposite modified electrodes
The above characterizations reveal that the CPE modified with electrochemically polymerized PEDOT/NCC nanocomposite has large electrode surface area and low electrochemical impedance, it is therefore expected that it may possess excellent electrocatalytic property. The electrochemical oxidation of DA at different electrodes was investigated using CV, as shown in Fig. 5. At the bare CPE (curve a), DA shows a couple of small redox peaks with a peak separation (ΔEp) of 466 mV, while at the CPE modified with PEDOT doped with perchlorate (curve b), DA presents larger redox peaks, and the ΔEp is decreased to about 209 mV. This result implies that PEDOT has good catalytic activity towards the oxidation of DA. For the CV of DA at the CPE modified with electrochemically polymerized PEDOT/NCC, a pair of well-defined redox peaks is observed (curve c), and the ΔEp is further decreased to about 150 mV. This clearly indicates that the catalytic activity of the PEDOT/NCC nanocomposite is superior than that of the commonly used PEDOT/perchlorate, which may be ascribed to the fact the nanocomposite's large surface area and good conductivity.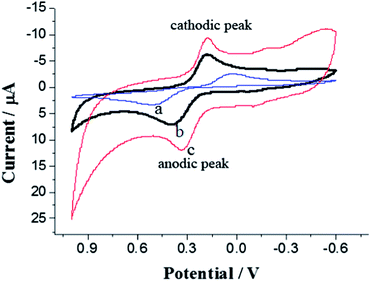 | ||
| Fig. 5 CV curves for 0.1 mM DA at (a) bare CPE, and CPEs modified with electropolymerized PEDOT doped with (b) perchlorate and (c) NCC in B–R buffer (0.2 M, pH 5.0). The scan rate was 100 mV s−1. | ||
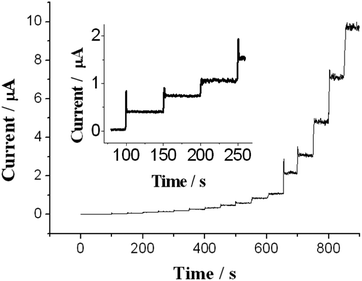
The amperometric response of DA on the CPE modified with the electrodeposited PEDOT/NCC was measured at an applied constant potential of 0.34 V, as shown in Fig. 6. The modified electrode shows a very fast catalytic response, and 95% of the steady state current is reached within 3 s. The sensor exhibits clear linear response to DA across a very wide concentration range from 0.2 to 62.0 μM, and the regression equation is Ipa (μA) = 1.558c (μM) − 0.959, with R = 0.998. Based on a signal-to-noise ratio of 3, the limit of detection of this assay is calculated to be 69 nM, which is lower than that of some related reports,47–50 indicating high sensitivity of the DA sensor.
Conclusions
Novel conducting polymer nanocomposites of PEDOT doped with NCC were successfully prepared by chemical oxidative polymerization and electrochemical polymerization, respectively. In both processes, NCC can be effectively doped in the conducting polymer PEDOT to form nanocomposites with large surface area and good conductivity. For the electrochemical polymerization of PEDOT, NCC serves as the sole dopant as well as a template to guide the formation of PEDOT around NCC nanorods. The electrodeposited PEDOT/NCC nanocomposite on CPE presented high surface area, low electrochemical impedance and excellent electrochemical catalytic activity. The amperometric sensor based on electrodeposited PEDOT/NCC achieved sensitive detection of DA (69 nM). In addition to these excellent properties, the low cost and facile preparation of PEDOT/NCC nanocomposite should make it an ideal candidate material for a broad range of applications in the future, such as the development of other sensing systems, on demand drug release and energy storage devices.Acknowledgements
This research was supported by the National Natural Science Foundation of China (21275087), the Natural Science Foundation of Shandong Province of China (ZR2012BM008), and the Taishan Scholar Program of Shandong Province, China. X.T.C. acknowledges the support of the National Science Foundation Grant 0748001.Notes and references
- J. Roncali, P. Blanchard and P. Frere, J. Mater. Chem., 2005, 15, 1589–1610 RSC.
- B. L. Groenedaal, F. Jonas, D. Freitag, H. Pielartzik and J. R. Reynolds, Adv. Mater., 2000, 12, 481–497 CrossRef.
- S. Bhandari, M. Deepa, A. K. Srivastava, A. G. Joshi and R. Kant, J. Phys. Chem. B, 2009, 113, 9416–9428 CrossRef CAS PubMed.
- X. Z. Liu, H. Q. Li, D. Li, M. Ishida and H. S. Zhou, J. Power Sources, 2013, 243, 374–380 CrossRef CAS PubMed.
- J. Yan, C. H. Sun, F. R. Tan, X. J. Hu, P. Chen, S. C. Qu, S. Y. Zhou and J. K. Xu, Sol. Energy Mater. Sol. Cells, 2010, 94, 390–394 CrossRef CAS PubMed.
- C. H. Lin, K. T. Chen, J. R. Ho, J. W. J. Cheng and R. C. C. Tsiang, J. Nanotechnol., 2012, 2012, 942629 Search PubMed.
- X. Yin, F. Wu, N. Q. Fu, J. Han, D. L. Chen, P. Xu, M. He and Y. Lin, ACS Appl. Mater. Interfaces, 2013, 5, 8423–8429 CAS.
- P. Lin, F. Yan and H. L. W. Chan, ACS Appl. Mater. Interfaces, 2010, 2, 1637–1641 CAS.
- X. L. Luo, C. L. Weaver, D. D. Zhou, R. Greenber and X. Y. T. Cui, Biomaterials, 2011, 32, 5551–5557 CrossRef CAS PubMed.
- S. Letaief, P. Aranda, R. Fernandez-Saavedra, J. C. (Jim) Margeson, C. Detellier and E. Ruiz-Hitzky, J. Mater. Chem., 2008, 18, 2227–2233 RSC.
- G. Y. Xu, B. B. Li, X. Y. T. Cui, L. Y. Ling and X. L. Luo, Sens. Actuators, B, 2013, 188, 405–410 CrossRef CAS PubMed.
- T. T. Tung, M. Castro, J. F. Feller, T. Y. Kim and K. S. Suh, Org. Electron., 2013, 14, 2789–2794 CrossRef CAS PubMed.
- L. M. Lu, O. Zhang, J. K. Xu, Y. P. Wen, X. M. Duan, H. M. Yu, L. P. Wu and T. Nie, Sens. Actuators, B, 2013, 181, 567–574 CrossRef CAS PubMed.
- J. J. He, N. W. Duffy, J. M. Pringle and Y. B. Cheng, Electrochim. Acta, 2013, 105, 275–281 CrossRef CAS PubMed.
- S. Radhakrishnan, C. Sumathi, A. Umar, S. J. Kim, J. Wilson and V. Dharuman, Biosens. Bioelectron., 2013, 47, 133–140 CrossRef CAS PubMed.
- Y. J. Yang, S. B. Li, L. N. Zhang, J. H. Xu, W. Y. Yang and Y. D. Jiang, ACS Appl. Mater. Interfaces, 2013, 5, 4350–4355 CAS.
- J. S. Fan and Y. H. Li, Carbohydr. Polym., 2012, 88, 1184–1188 CrossRef CAS PubMed.
- S. Elazzouzi-Hafraoui, Y. Nishiyama, J. L. Putaux, L. Heux, F. Dubreuil and C. Rochas, Biomacromolecules, 2008, 9, 57–65 CrossRef CAS PubMed.
- B. L. Peng, N. Dhar, H. L. Liu and K. C. Tam, Can. J. Chem. Eng., 2011, 89, 1191–1206 CrossRef CAS.
- J. K. Jackson, K. Letchford, B. Z. Wasserman, L. Ye, W. Y. Hamad and H. M. Burt, Int. J. Nanomed., 2011, 6, 321–330 CAS.
- E. Lam, K. B. Male, J. H. Chong, A. C. Leung and J. H. Luong, Trends Biotechnol., 2012, 30, 283–290 CrossRef CAS PubMed.
- Y. Habibi, L. A. Lucia and O. J. Rojas, Chem. Rev., 2010, 110, 3479–3500 CrossRef CAS PubMed.
- J. H. Pan, W. Hamad and S. K. Straus, Macromolecules, 2010, 43, 3851–3858 CrossRef CAS.
- E. Fortunati, M. Peltzer, I. Armentano, A. Jiménez and J. M. Kenny, Food Eng., 2013, 118, 117–124 CrossRef CAS PubMed.
- N. Follain, S. Belbekhouche, J. Bras, G. Siqueira, S. Marais and A. Dufresne, J. Membr. Sci., 2013, 427, 218–229 CrossRef CAS PubMed.
- C. J. Zhou, Q. L. Wu, Y. Y. Yue and Q. G. Zhang, J. Colloid Interface Sci., 2011, 353, 116–123 CrossRef CAS PubMed.
- V. Incani, C. Danumah and Y. Boluk, Cellulose, 2013, 20, 191–200 CrossRef CAS.
- J. V. Edwards, N. Prevost, K. Sethumadhavan, A. Ullah and B. Condon, Cellulose, 2013, 20, 1223–1235 CrossRef CAS.
- H. Liu, D. Wang, Z. Q. Song and S. B. Shang, Cellulose, 2011, 18, 67–74 CrossRef CAS.
- Y. P. Zhang, V. P. Chodavarapu, A. G. Kirk and M. P. Andrews, Sens. Actuators, B, 2013, 176, 692–697 CrossRef CAS PubMed.
- A. Dong-Hyun Lee and Y. Ning, Master thesis, Faculty of Forestry University of Toronto, 2011.
- M. A. S. A. Samir, F. Alloin, J. Y. Sanchez and A. Dufresne, Polymer, 2004, 45, 4149–4157 CrossRef PubMed.
- A. Kaboorani, B. Riedl, P. Blanchet, M. Fellin, O. Hosseinaei and S. Wang, Eur. Polym. J., 2012, 48, 1829–1837 CrossRef CAS PubMed.
- A. H. Pei, Q. Zhou and L. A. Berglund, Compos. Sci. Technol., 2010, 70, 815–821 CrossRef CAS PubMed.
- S. A. Paralikar, J. Simonsen and J. Lombardi, J. Membr. Sci., 2008, 320, 248–258 CrossRef CAS PubMed.
- M. S. Peresin, Y. Habibi, J. O. Zoppe, J. J. Pawlak and O. J. Rojas, Biomacromolecules, 2010, 11, 674–681 CrossRef CAS PubMed.
- D. W. Zhang, L. H. Zhang, B. Z. Wang and G. Z. Piao, J. Mater., 2013, 2013, 1–6 Search PubMed.
- G. Nyström, A. Razaq, M. Strømme, L. Nyholm and A. Mihranyan, Nano Lett., 2009, 9, 3635–3639 CrossRef PubMed.
- G. Y. Xu, B. B. Li and X. L. Luo, Sens. Actuators, B, 2013, 176, 69–74 CrossRef CAS PubMed.
- Z. X. Zheng, Y. L. Du, Q. L. Feng, Z. H. Wang and C. M. Wang, J. Mol. Catal. A: Chem., 2012, 353–354, 80–86 CrossRef CAS PubMed.
- J. Singh, A. Roychoudhury, M. Srivastava, V. Chaudhary, R. Prasanna, D. W. Lee, S. H. Lee and B. D. Malhotra, J. Phys. Chem. C, 2013, 117, 8491–8502 CAS.
- A. Periyakaruppan, P. U. Arumugam, M. Meyyappan and J. E. Koehne, Biosens. Bioelectron., 2011, 28, 428–434 CrossRef CAS PubMed.
- A. Periyakaruppan, R. P. Gandhiraman, M. Meyyappan and J. E. Koehne, Anal. Chem., 2013, 85, 3858–3863 CrossRef CAS PubMed.
- D. Aradilla, F. Estrany, D. S. Azambujad, M. T. Casasa, J. Puiggali, C. A. Ferreirae and C. Alemán, Eur. Polym. J., 2010, 46, 977–983 CrossRef CAS PubMed.
- Y. Yao, N. Liu, M. T. McDowell, M. Pasta and Y. Cui, Energy Environ. Sci., 2012, 5, 7927–7930 CAS.
- J. Y. Yang, D. H. Kim, J. L. Hendricks, M. Leach, R. Northey and D. C. Maritin, Acta Biomater., 2005, 1, 125–136 CrossRef PubMed.
- K. K. Liu, H. Y. Pang, J. R. Zhang, H. P. Huang, Q. Liu and Y. H. Chu, RSC Adv., 2014, 4, 8415–8420 RSC.
- E. Scavetta, R. Mazzoni, F. Mariani, R. G. Margutta, A. Bonfiglio, M. Demelas, S. Fiorilli, M. Marzocchi and B. Fraboni, J. Mater. Chem. B, 2014, 2, 2861–2867 RSC.
- S. Lupu, C. Lete, P. C. Balaure, F. J. D. Campo, F. X. Munoz, B. Lakard and J. Y. Hihn, Sens. Actuators, B, 2013, 181, 136–143 CrossRef CAS PubMed.
- S. Lupu, C. Lete, P. C. Balaure, D. I. Caval, C. Mihailciuc, B. Lakard, J. Y. Hihn and F. J. D. Campo, Sensors, 2013, 13, 6759–6774 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |