Coumarin based dual switching fluorescent ‘turn-on’ chemosensor for selective detection of Zn2+ and HSO4−: an experimental and theoretical study†
Deblina Sarkar,
Ajoy Kumar Pramanik and
Tapan Kumar Mondal*
Department of Chemistry, Jadavpur University, Kolkata-700032, India. E-mail: tkmondal@chemistry.jdvu.ac.in
First published on 29th May 2014
Abstract
A highly sensitive and selective coumarin based fluorescence ‘turn-on’ chemosensor (HL) for the efficient detection of Zn2+ and HSO4− over other ions has been developed. Theoretical study interprets the electronic structure and sensing properties of the developed HL.
Introduction
Development of artificial optical chemosensors for recognizing biologically and environmentally important analytes has become an active field of research1 with outstanding potential applications in the fields of analytical chemistry,2 and clinical biology including environmental studies.3 These molecules with photoinduced switching properties have a great deal of application in the fields of molecular electronics and sensor device applications.4 However most of the reported chemosensors are effective only in selective recognition of a particular analyte.5 Thus development of multifunctional chemosensors for the detection of more than one analyte still remains a challenging field of research.6Zinc is the second most abundant and essential element in human body with active participation in various biologically essential processes like oxygen transport,7 gene expression,8 energy generation9 and cellular metabolism.10 But unregulated level of Zn2+ in human body may lead to several life threatening diseases such as β-thalassemia, Friedreich's ataxia, and several neurodegenerative diseases including Alzheimer's disease, Parkinson's disease and epilepsy.11 Again Zn2+ is also a harmful pollutant for our environment.12 Thus it is highly important to develop artificial chemosensors for the efficient detection of Zn2+ both ‘in vivo’ as well as ‘in vitro’ cases. Due to this immense demand in the field of development of artificial chemosensors for Zn2+, several sensing devices comprising of Schiff base skeleton, polythiacrown ethers, have been developed in the recent few years.13 However most of the artificial fluorescent probes used for detection of Zn2+ suffer the problem of insufficient selectivity due to special interference from Cd2+.14 FluoZin-3, a commonly used Zn2+ sensor suffers the problem of interference from Ca2+ at higher concentratiom.15
Recognition and sensing of HSO4− is specially an active field of research as this anion can be found in agricultural fertilizers and industrial raw materials which can affect the environment adversely.16 HSO4− dissociates at higher pH producing sulphate ion which causes skin problems, irritation to eyes and also creates respiratory problems. HSO4− ion can also be used for preventing environmental damage caused due to radioactive waste.17 HSO4− also plays an important role in biological systems18 but till date only a few chemosensors for the detection of this anion have been reported so far.19
Thus herein we report a coumarin based chemosensor showing dual switching ‘turn-on’ property for both Zn2+ and HSO4−. Coumarin framework has been used as the basic fluorophore due to its various interesting photophysical properties such as Strokes shift and visible excitation and emission wavelengths.20 Moreover coumarin moiety has high importance as fluorescent dyes.21 Gradual addition of Zn2+ to HL in CH3CN–H2O, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v (at 25 °C) shows an excellent fluorescence emission intensity enhancement of 27 fold. Whereas in presence of HSO4−, HL shows an emission intensity increase of 17 fold. The synthesized chemosensor HL is highly selective towards Zn2+ even in presence of other metal ions and is also highly selective for HSO4− over other anions. Moreover the receptor HL can also act as a water soluble fluorescent sensor for HSO4−. Only a few water soluble chemosensors have been reported so far for HSO4−.22
1, v/v (at 25 °C) shows an excellent fluorescence emission intensity enhancement of 27 fold. Whereas in presence of HSO4−, HL shows an emission intensity increase of 17 fold. The synthesized chemosensor HL is highly selective towards Zn2+ even in presence of other metal ions and is also highly selective for HSO4− over other anions. Moreover the receptor HL can also act as a water soluble fluorescent sensor for HSO4−. Only a few water soluble chemosensors have been reported so far for HSO4−.22
Results and discussion
Synthesis and spectral characterization
Synthetic route towards receptor HL involves a very facile and economically cheap route involving the Schiff base condensation of 3-acetyl-4-hydroxycoumarin and N,N′-dimethyl-p-phenylenediamine in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio in methanolic medium under reflux condition. Excellent yield of 77% has been obtained (Scheme 1). The receptor HL has been characterized by elemental and mass spectral analysis along with several other spectroscopic techniques (IR, UV-vis, NMR etc.).
1 molar ratio in methanolic medium under reflux condition. Excellent yield of 77% has been obtained (Scheme 1). The receptor HL has been characterized by elemental and mass spectral analysis along with several other spectroscopic techniques (IR, UV-vis, NMR etc.).
In the IR spectrum, the lactone C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching frequency appears at 1709 cm−1, keto C
O stretching frequency appears at 1709 cm−1, keto C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O appears at 1616 cm−1 and C
O appears at 1616 cm−1 and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C appears at 1568 cm−1. In 1H NMR spectrum of the sensor HL in CDCl3, hydrogen bonded NH proton appears at δ 15.58 (ref. 23) which disappears in the HL–Zn2+ complex due to coordination of HL to the metal in the enol form. The aromatic protons in HL appear as expected in the region δ 8.08–6.73. The –COCH3 protons appear at δ 2.68 as singlet (Fig. S1†) and the –NCH3 appear at δ 3.01 as singlet as expected. In the HL–Zn2+ complex all aromatic protons appear at a bit downfield position compared to that of HL, which can be clearly explained due to the coordination of Zn2+ with HL. 13C NMR spectrum of HL shows a peak at δ 181.5 corresponding to the keto C
C appears at 1568 cm−1. In 1H NMR spectrum of the sensor HL in CDCl3, hydrogen bonded NH proton appears at δ 15.58 (ref. 23) which disappears in the HL–Zn2+ complex due to coordination of HL to the metal in the enol form. The aromatic protons in HL appear as expected in the region δ 8.08–6.73. The –COCH3 protons appear at δ 2.68 as singlet (Fig. S1†) and the –NCH3 appear at δ 3.01 as singlet as expected. In the HL–Zn2+ complex all aromatic protons appear at a bit downfield position compared to that of HL, which can be clearly explained due to the coordination of Zn2+ with HL. 13C NMR spectrum of HL shows a peak at δ 181.5 corresponding to the keto C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O carbon while at δ 175.6 the peak corresponds to the C–N carbon, and at δ 162.6 corresponds to lactone carbon in the coumarin ring. The –NCH3 carbon appears at δ 40.4 while the acetyl carbon appears at δ 20.8 (Fig. S2†). Mass spectra show m/z peak corresponding to [M + H]+ at 323.2 for HL (Fig. S3†) and at 461.2 corresponding to [M + Na]+ for the HL–Zn2+ complex (Fig. S4†) thus supporting 1
O carbon while at δ 175.6 the peak corresponds to the C–N carbon, and at δ 162.6 corresponds to lactone carbon in the coumarin ring. The –NCH3 carbon appears at δ 40.4 while the acetyl carbon appears at δ 20.8 (Fig. S2†). Mass spectra show m/z peak corresponding to [M + H]+ at 323.2 for HL (Fig. S3†) and at 461.2 corresponding to [M + Na]+ for the HL–Zn2+ complex (Fig. S4†) thus supporting 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex formation.
1 complex formation.
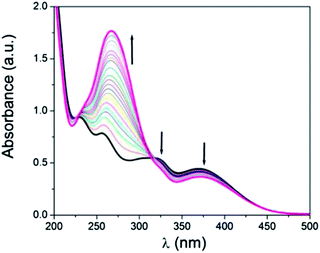
Cation sensing studies of HL
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v CH3CN–H2O using HEPES buffered solution at pH = 7.2. Upon gradual addition of ZnCl2 (100 μM) solution the band at 256 nm shifts and a new strong absorption band appears at 267 nm. The absorption band at 321 nm and 369 nm gradually decreases on addition of Zn2+ and a distinct isosbestic point appears at 314 nm. This red shift and gradual decrease in absorption intensity is due to the coordination of HL to Zn2+ (Fig. 1). UV-vis spectrum of HL is also studied in presence of other metal ions i.e. Na+, K+, Ca2+, Mg2+, Mn2+, Fe3+, Cr3+, Al3+, Co2+, Ni2+, Cu2+, Cd2+ and Hg2+ but there is hardly any change in the spectral pattern (Fig. S5†).
1, v/v CH3CN–H2O using HEPES buffered solution at pH = 7.2. Upon gradual addition of ZnCl2 (100 μM) solution the band at 256 nm shifts and a new strong absorption band appears at 267 nm. The absorption band at 321 nm and 369 nm gradually decreases on addition of Zn2+ and a distinct isosbestic point appears at 314 nm. This red shift and gradual decrease in absorption intensity is due to the coordination of HL to Zn2+ (Fig. 1). UV-vis spectrum of HL is also studied in presence of other metal ions i.e. Na+, K+, Ca2+, Mg2+, Mn2+, Fe3+, Cr3+, Al3+, Co2+, Ni2+, Cu2+, Cd2+ and Hg2+ but there is hardly any change in the spectral pattern (Fig. S5†).
 | ||
Fig. 2 Changes in emission spectrum of HL (10 μM) upon gradual addition of Zn2+ (100 μM) (λexcittion = 370 nm) in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v CH3CN–H2O using HEPES buffered solution at pH = 7.2. 1, v/v CH3CN–H2O using HEPES buffered solution at pH = 7.2. | ||
Jobs plot of emission intensity shows a maxima in the plot corresponds to ∼0.5 mole fraction indicating 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex formation of HL with Zn2+ (Fig. S7†). From emission spectral change, limit of detection of the chemosensor for Zn2+ is determined using the equation LOD = K × SD/S where SD is the standard deviation of the blank solution and S in the slope of the calibration curve (Fig. S8†). The limit of detection for Zn2+ is found to be 6.541 × 10−5 M from fluorescent spectral titration. This result clearly demonstrates that the chemosensor is highly efficient in sensing Zn2+ even in very minute level. From fluorescent spectral titration the association constant of HL with Zn2+ is found to be 4.8 × 105 and stoichiometry of the reaction n = 1.113 indicating 1
1 complex formation of HL with Zn2+ (Fig. S7†). From emission spectral change, limit of detection of the chemosensor for Zn2+ is determined using the equation LOD = K × SD/S where SD is the standard deviation of the blank solution and S in the slope of the calibration curve (Fig. S8†). The limit of detection for Zn2+ is found to be 6.541 × 10−5 M from fluorescent spectral titration. This result clearly demonstrates that the chemosensor is highly efficient in sensing Zn2+ even in very minute level. From fluorescent spectral titration the association constant of HL with Zn2+ is found to be 4.8 × 105 and stoichiometry of the reaction n = 1.113 indicating 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex formation (Fig. S9†).
1 complex formation (Fig. S9†).
Fluorescence emission intensity of HL (10 μM) is studied in presence of other metals i.e. Na+, K+, Ca2+, Mg2+, Mn2+, Fe3+, Cr3+, Al3+, Co2+, Ni2+, Cu2+, Cd2+ and Hg2+ (100 μM) in CH3CN–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, pH = 7.2) but there is hardly any increase in emission intensity of HL (Fig. 3). Then to these solutions Zn2+ is added which then shows an instant fluorescent enhancement (Fig. S10†). Thus the synthesized receptor HL is highly efficient in detection of Zn2+ in presence of other metals and thus it can detect Zn2+ in biological or environmental samples where other metals usually co-exist with Zn2+.
1, v/v, pH = 7.2) but there is hardly any increase in emission intensity of HL (Fig. 3). Then to these solutions Zn2+ is added which then shows an instant fluorescent enhancement (Fig. S10†). Thus the synthesized receptor HL is highly efficient in detection of Zn2+ in presence of other metals and thus it can detect Zn2+ in biological or environmental samples where other metals usually co-exist with Zn2+.
Anion sensing study
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex formation of HL with HSO4− (Fig. S14†). The emission intensity of HL with HSO4− in presence of several other anions has been studied and in every case there is no significant interference due to the presence of other anions (Fig. S15†).
1 complex formation of HL with HSO4− (Fig. S14†). The emission intensity of HL with HSO4− in presence of several other anions has been studied and in every case there is no significant interference due to the presence of other anions (Fig. S15†).
Electronic structure and DFT calculation
To interpret the electronic structure of HL, geometry optimization has been performed by DFT/B3LYP method in singlet ground state (S0) (Fig. 6). The potential energy scan (Fig. 7) in S0 state reveals that the keto form is more stable by an amount of energy of 7.15 kcal mol−1 than the corresponding enol form which is consistent with the X-ray structure of this type of molecules.23 The geometry of HL–Zn2+ and HL–HSO4− have been optimized and the energy minimized structures are shown in Fig. 8 and 9 respectively. Contour plot of selected molecular orbitals of HL and its complexes with Zn2+ and HSO![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) are given in Fig. S16, S17 and S18† respectively. HL acts as a N, O bidentate ligand and gets coordinated to Zn2+ in a tetrahedral fashion with the other two sites occupied by a water molecule and chloride ion. In HL–HSO4−, hydrogen atom of HSO4− gets hydrogen bonded with the imine-N of HL with a distance of 1.78 Å. The O atom of HSO4− gets H bonded with OH of coumarin ring with a distance of 1.58 Å.
are given in Fig. S16, S17 and S18† respectively. HL acts as a N, O bidentate ligand and gets coordinated to Zn2+ in a tetrahedral fashion with the other two sites occupied by a water molecule and chloride ion. In HL–HSO4−, hydrogen atom of HSO4− gets hydrogen bonded with the imine-N of HL with a distance of 1.78 Å. The O atom of HSO4− gets H bonded with OH of coumarin ring with a distance of 1.58 Å.
Experimental section
Material and methods
4-Hydroxycoumarin and N,N-dimethylbenzene-1,4-diamine were purchased from Aldrich. All other organic chemicals and inorganic salts were available from commercial suppliers and used without further purification.Elemental analysis was carried out in a 2400 Series-II CHN analyzer, Perkin Elmer, USA. ESI mass spectra were recorded on a micromass Q-TOF mass spectrometer. Infrared spectra were taken on a RX-1 Perkin Elmer spectrophotometer with samples prepared as KBr pellets. Electronic spectral studies were performed on a Perkin Elmer Lambda 25 spectrophotometer. Luminescence property was measured using Perkin Elmer LS 55 fluorescence spectrophotometer at room temperature (298 K). NMR spectra were recorded using a Bruker (AC) 300 MHz FTNMR spectrometer in CDCl3. Fluorescence lifetimes were measured using a time-resolved spectrofluorometer from IBH, UK. The instrument uses a picoseconds diode laser (NanoLed-03, 370 nm) as the excitation source and works on the principle of time-correlated single photon counting.25 The goodness of fit was evaluated by χ2 criterion and visual inspection of the residuals of the fitted function to the data.
The luminescence quantum yield was determined using carbazole as reference with a known ϕR of 0.42 in MeCN. The complex and the reference dye were excited at the same wavelength, maintaining nearly equal absorbance (∼0.1), and the emission spectra were recorded. The area of the emission spectrum was integrated using the software available in the instrument and the quantum yield is calculated according to the following equation:
| ϕS/ϕR = [AS/AR ] × [(Abs)R /(Abs)S ] × [ηS2/ηR2]. |
Synthesis of 3-[1-(4-dimethylamino)phenylimino]ethyl-4-hydroxy-2H-chromen-2-one (HL)
3-Acetyl-4-hydroxy-2H-chromen-2-one (L)26 (0.306 g, 1.5 mmol) and N,N′-dimethylbenzene-1,4-diamine (0.204 g, 1.5 mmol) were refluxed for 10 hours in methanolic medium. Excess solvent was evaporated under reduced pressure and then dissolved in dichoromethane which is further subjected to silica gel (60–120 mesh) column chromatographic separation. The desired light yellow solid product was obtained by elution with 20% ethylacetate–petether (v/v) mixture. Yield: 0.371 g, 77%.Anal. calc. for C19H18N2O3 (HL): calc. (%) C 70.79, H 5.63, N 8.69. Found (%), C 70.93, H 5.59, N 8.52. IR data (KBr, cm−1): 1709 ν(lactone C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1616 ν(keto C
O); 1616 ν(keto C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1568 ν(C
O), 1568 ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C). 1H NMR data (CDCl3, 300 MHz): δ 15.58 (1H, s), 8.08 (1H, d, J= 7.7 Hz), 7.53–7.55 (1H, m), 7.22–7.25(1H, m), 7.07 (1H, d, J = 8.8 Hz), 6.73 (1H, d, J= 8.9 Hz), 3.01 (6H, s), 2.68 (3H, s). 13C NMR (CDCl3, 75 MHz): δ 181.5, 175.6, 162.6, 153.8, 149.9, 133.8, 126.1, 126.0, 124.7, 123.5, 120.3, 116.6, 112.4, 97.8, 40.4, 20.8. Melting point: 205 °C.
C). 1H NMR data (CDCl3, 300 MHz): δ 15.58 (1H, s), 8.08 (1H, d, J= 7.7 Hz), 7.53–7.55 (1H, m), 7.22–7.25(1H, m), 7.07 (1H, d, J = 8.8 Hz), 6.73 (1H, d, J= 8.9 Hz), 3.01 (6H, s), 2.68 (3H, s). 13C NMR (CDCl3, 75 MHz): δ 181.5, 175.6, 162.6, 153.8, 149.9, 133.8, 126.1, 126.0, 124.7, 123.5, 120.3, 116.6, 112.4, 97.8, 40.4, 20.8. Melting point: 205 °C.
General method for UV-vis and fluorescence titration
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v] (at 25 °C) using HEPES buffered solution (50 mM) at pH = 7.2 was prepared. The solution of the guest cations and anions using their chloride salts and sodium salts respectively in the order of 10−4 M were prepared in deionised water. Solutions of various concentrations containing host and increasing concentrations of cations and anions were prepared separately. The spectra of these solutions were recorded by means of UV-vis method.
1, v/v] (at 25 °C) using HEPES buffered solution (50 mM) at pH = 7.2 was prepared. The solution of the guest cations and anions using their chloride salts and sodium salts respectively in the order of 10−4 M were prepared in deionised water. Solutions of various concentrations containing host and increasing concentrations of cations and anions were prepared separately. The spectra of these solutions were recorded by means of UV-vis method.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) was used as solvent at pH 7.2 using HEPES buffer. Again a series of solutions containing HL (10 μM) and NaHSO4 (10 μM) were prepared such that the sum of the total volume remained constant (4 ml). Here also CH3CN–H2O (1
1, v/v) was used as solvent at pH 7.2 using HEPES buffer. Again a series of solutions containing HL (10 μM) and NaHSO4 (10 μM) were prepared such that the sum of the total volume remained constant (4 ml). Here also CH3CN–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) was used as solvent at pH 7.2 using HEPES buffer. Job's plots were drawn by plotting ΔF versus mole fraction of Zn2+ or HSO4− [ΔF = change of intensity of the emission spectrum at 457 nm (for Zn2+) and at 376 nm (for HSO4−) during titration and Xg is the mole fraction of the guest in each case, respectively].
1, v/v) was used as solvent at pH 7.2 using HEPES buffer. Job's plots were drawn by plotting ΔF versus mole fraction of Zn2+ or HSO4− [ΔF = change of intensity of the emission spectrum at 457 nm (for Zn2+) and at 376 nm (for HSO4−) during titration and Xg is the mole fraction of the guest in each case, respectively].Conclusion
Thus in brief we report herein a coumarin based organic moety which shows dual sensing fluorescent ‘turn-on’ property in presence of both Zn2+ and HSO4− ions. The developed chemosensor is synthesized using a very simple and economically cheap synthetic route with excellent yield. The developed chemosensor shows high selectivity for Zn2+ and HSO4− even in presence of several other ions. We believe that in near future our developed chemosensor HL will be useful in designing more selective and efficient receptors for recognizing Zn2+ in biological systems.Acknowledgements
The authors thank Department of Science and Technology India, New Delhi, India for their financial support. D. Sarkar and A. K. Pramanik thank CSIR, India for their fellowship. Jadavpur University is acknowledged for providing infrastructural facility.Notes and references
- (a) Z. Kösterel and K. Severin, Chem. Commun., 2012, 48, 5841 RSC; (b) M. Zhang and B. C. Ye, Chem. Commun., 2012, 48, 3647 RSC; (c) J. F. Callan, A. P. de Silva and D. C. Magri, Tetrahedron, 2005, 61, 8551 CrossRef CAS PubMed; (d) R. Martinez-Manez and F. Sancenon, Chem. Rev., 2003, 103, 4419 CrossRef CAS PubMed; (e) A. P. de Silva, H. Q. N. Gunaratne, T. Gunnlaugsson, A. J. M. Huxley, C. P. McCoy, J. T. R. Rademache and T. E. Rice, Chem. Rev., 1997, 97, 1515 CrossRef CAS PubMed.
- (a) M. Vonlanthen and N. S. Finney, J. Org. Chem., 2013, 78, 3980 CrossRef CAS PubMed; (b) V. A. Babain, A. V. Legin, D. O. Kirsanov, A. M. Rudnitskaya, Y. M. Tatuev and V. E. Baulin, Radiochim. Acta, 2009, 98, 479 Search PubMed; (c) H. Hisamoto, H. Tohma, T. Yamada, K. Yamuchi, D. Siswanta, N. Yoshioka and K. Suzuki, Anal. Chim. Acta, 1998, 373, 271 CrossRef CAS.
- (a) S. E. Martinez, C. L. Sayre and N. M. Davies, Biomed. Chromatogr., 2013, 27, 67 CrossRef CAS PubMed; (b) Z. Wang, D. M. Han, W. P. Jia, Q. Z. Zhou and W. P. Deng, Anal. Chem., 2012, 84, 4915 CrossRef CAS PubMed; (c) M. Alexy, M. Hanko, S. Rentmeister and J. Heinze, Sens. Actuators, B, 2006, 114, 916 CrossRef CAS PubMed.
- (a) M. V. Alfimov, O. A. Fedorova and S. P. Gromov, J. Photochem. Photobiol., A, 2003, 183, 2 Search PubMed; (b) M. Irie, Chem. Rev., 2000, 5, 1683 CrossRef PubMed.
- (a) Q. Qu, A. Zhu, X. Shao, G. Shib and Y. Tian, Chem. Commun., 2012, 48, 5473 RSC; (b) K. Ghosh, T. Sarkara and A. Samadderb, Org. Biomol. Chem., 2012, 10, 3236 RSC; (c) J. Zhang, C. Yu, G. Lu, Q. Fu, N. Li and Y. Ji, New J. Chem., 2012, 36, 819 RSC; (d) C. Lodeiro and F. Pina, Coord. Chem. Rev., 2009, 253, 1353 CrossRef CAS PubMed; (e) L. Basabe-Desmonts, D. N. Reinhoudt and M. Crego-Calama, Chem. Soc. Rev., 2007, 36, 993 RSC; (f) P. A. Gale, Acc. Chem. Res., 2006, 39, 465 CrossRef CAS PubMed; (g) E. A. Katayev, Y. A. Ustynyuk and J. L. Sessler, Coord. Chem. Rev., 2006, 250, 3004 CrossRef CAS PubMed.
- (a) Z. Guo, I. Shin and J. Yoon, Chem. Commun., 2012, 48, 5956 RSC; (b) H. Sharma, N. Kaur and N. Singh, Inorg. Chim. Acta, 2012, 391, 83 CrossRef CAS PubMed; (c) T. Konry and D. R. Walt, J. Am. Chem. Soc., 2009, 131, 13232 CrossRef CAS PubMed; (d) N. Kaur and S. Kumar, Tetrahedron Lett., 2008, 49, 5067 CrossRef CAS PubMed; (e) M. Schmittel and H. W. Lin, Angew. Chem., Int. Ed., 2007, 46, 893 CrossRef CAS PubMed; (f) H. Komatsu, T. Miki, D. Citterio, T. Kubota, Y. Shindo, Y. Kitamura, K. Oka and K. Suzuki, J. Am. Chem. Soc., 2005, 127, 10798 CrossRef CAS PubMed; (g) H. Komatsu, D. Citterio, Y. Fujiwara, K. Minamihashi, Y. Araki, M. Hagiwara and K. Suziki, Org. Lett., 2005, 7, 2857 CrossRef CAS PubMed; (h) D. Jimenez, F. Martinez-Manez, F. Sancenon and J. Soto, Tetrahedron Lett., 2004, 76, 5726 Search PubMed.
- E. Loisel, L. Jacquamet, L. Serre, C. Bauvois, J. L. Ferrer, T. Vernet, A. M. D. Guilmi and C. Duurmort, J. Mol. Biol., 2008, 381, 594 CrossRef CAS PubMed.
- R. A. Sperotto, T. Boff, G. L. Duarte, L. S. Santos, M. A. Grusak and J. P. Fett, J. Plant Physiol., 2010, 167, 1500 CrossRef CAS PubMed.
- H. Yang, Y. Peng, L. Haung, H. Zhang, Y. Wang and S. Xie, J. Lumin., 2013, 135, 26 CrossRef CAS PubMed.
- D. J. Bobilya, J. T. Reynolds, K. L. Faia, M. B. Anderson and P. G. Reeves, J. Nutr. Biochem., 1999, 10, 139 CrossRef CAS.
- (a) J. Lai, A. Moxey, G. Nowak, K. Vashum, K. Bailey and M. McEvoy, J. Affective Disord., 1999, 56, 189 CrossRef; (b) J. H. Weiss, S. L. Sensi and J. Y. Koh, Trends Pharmacol. Sci., 2000, 21, 395 CrossRef CAS; (c) M. Maes, N. D. Vos, P. Demedts, A. Wauters and H. Neels, J. Affective Disord., 2012, 136, 31 CrossRef PubMed.
- (a) E. Zhang, E. Lui, J. Shen, Y. Cao and Y. Li, J. Environ. Sci., 2012, 24, 1189 CrossRef CAS; (b) J. Cherif, C. Mediouni, W. B. Ammar and F. Jemal, J. Environ. Sci., 2011, 23, 837 CrossRef CAS.
- (a) D. B. Kevin, V. B. Mykhailo, F. Andrew, R. M. Alma, D. K. Oleksiy, A. M. Ivan, E. M. Artem and V. P. Olga, J. Phys. Chem. B, 2010, 114, 9313 CrossRef PubMed; (b) H. M. Chawla, S. Richa and P. Shubha, Tetrahedron Lett., 2012, 53, 2996 CrossRef CAS PubMed; (c) T. B. Wei, P. Zhang, B. B. Shi, P. Chen, Q. Lin, J. Liu and Y. M. Zhang, Dyes Pigm., 2013, 97, 297 CrossRef CAS PubMed.
- (a) E. Kimura and T. Koike, Chem. Soc. Rev., 1998, 27, 179 RSC; (b) P. Jiang and Z. Guo, Coord. Chem. Rev., 2004, 248, 205 CrossRef CAS PubMed; (c) N. C. Lim, H. C. Freake and C. Bruckner, Chem.–Eur. J., 2005, 11, 38 CrossRef PubMed; (d) Z. C. Liu, B. D. Wang, Z. Y. Yang, T. R. Li and Y. Li, Inorg. Chem. Commun., 2010, 13, 606 CrossRef CAS PubMed.
- (a) A. R. Kay and J. Neur, Science, 2003, 23, 6847 CAS; (b) J. Zhao, B. A. Bertoglio, K. R. Gee and A. R. Kay, Cell Calcium, 2008, 44, 422 CrossRef CAS PubMed; (c) G. K. Walkup, S. C. Burdette, S. J. Lippard and R. Y. Tsien, J. Am. Chem. Soc., 2000, 122, 5644 CrossRef CAS; (d) S. C. Burdette, C. J. Frederickson, W. M. Bu and S. J. Lippard, J. Am. Chem. Soc., 2003, 125, 1778 CrossRef CAS PubMed; (e) E. M. Nolan, S. C. Burdette, J. H. Harvey, S. A. Hilderbrand and S. J. Lippard, Inorg. Chem., 2004, 43, 2624 CrossRef CAS PubMed; (f) C. J. Chang, E. M. Nolan, J. Jaworski, S. C. Burdette, M. Sheng and S. J. Lippard, Chem. Biol., 2004, 11, 203 CrossRef CAS PubMed.
- B. A. Moyer, L. H. Delmau, C. J. Fowler, A. Ruas, D. A. Bostick, J. L. Sessler, E. Katayeu, G. D. Pantos, J. M. Llinares, M. A. Hossain, S. O. Kang and K. Bowman-James, in Advances in Inorganic Chemistry, ed. R. V. Eldik and K. Bowman-James, Academic Press, New York, 2006, pp. 175–204 Search PubMed.
- B. A. Moyer, L. H. Delmau, C. J. Fowler, A. Ruas, D. A. Bostick, J. L. Sessler, E. Katayev, G. D. Pantos, J. M. Llinares, M. A. Hossain, S. O. Kang and K. Bowman-James, in Advances in Inorganic Chemistry: Template Effects and Molecular Organisation, ed. R. Van Eldik and K. Bowman-James, Academia Press, London, 2007, vol. 59, p. 175 Search PubMed.
- J. W. Pflugrath and F. A. Quiocho, Nature, 1985, 314, 257 CrossRef CAS.
- (a) S. Goswami, S. Das, K. Aich, D. Sarkar and T. K. Mondal, Tetrahedron Lett., 2013, 54, 6892 CrossRef CAS PubMed; (b) S. Goswami, A. K. Das, K. Aich, A. Manna, S. Maity, K. Khanrab and N. Bhattacharyya, Analyst, 2013, 138, 4593 RSC; (c) S. T. Yang, D. J. Liao, S. J. Chen, C. H. Hu and A. T. Wu, Analyst, 2012, 137, 1553 RSC; (d) W. J. Xue, L. Li, Q. Li and A. X. Wu, Talanta, 2012, 88, 734 CrossRef CAS PubMed; (e) P. Li, Y. M. Zhang, Q. Lin, J. Q. Li and T. B. Wei, Spectrochim. Acta, Part A, 2012, 90, 152 CrossRef CAS PubMed; (f) H. J. Kim, S. Bhuniya, R. K. Mahajan, R. Puri, H. G. Liu, K. C. Ko, J. Y. Lee and J. S. Kim, Chem. Commun., 2009, 7128 RSC; (g) R. Shen, X. B. Pan, H. F. Wang, L. H. Yao, J. C. Wu and N. Tang, Dalton Trans., 2008, 3574 RSC; (h) J. L. Sessler, E. Katayev, G. D. Pantosa and Y. A. Ustynyuk, Chem. Commun., 2004, 1276 RSC; (i) A. Mallick, T. Katayama, Y. Ishibasi, M. Yasudab and H. Miyasaka, Analyst, 2011, 136, 275 RSC; (j) M. Alfonso, A. Tarraga and P. Molina, Org. Lett., 2011, 13, 6432 CrossRef CAS PubMed.
- D. Ray and P. K. Bharadwaj, Inorg. Chem., 2008, 47, 2252 CrossRef CAS PubMed.
- (a) N. C. Lim, J. V. Schuster, M. C. Porto, M. A. Tanudra, L. L. Yao, H. C. Freake and C. Bruckner, Inorg. Chem., 2005, 44, 2018 CrossRef CAS PubMed; (b) H. E. Katerinopoulos, Curr. Pharm. Des., 2004, 10, 3835 CrossRef CAS.
- (a) P. Li, Y. M. Zhang, Q. Lin, J. Q. Li and T. B. Wei, Spectrochim. Acta, Part A, 2012, 90, 152 CrossRef CAS PubMed; (b) H. J. Kim, S. Bhuniya, R. K. Mahajan, R. Puri, H. G. Liu, K. C. Ko, J. Y. Lee and J. S. Kim, Chem. Commun., 2009, 7128 RSC; (c) M. Alfonso, A. Tarraga and P. Molina, Org. Lett., 2011, 13, 6432 CrossRef CAS PubMed.
- (a) A. Brahmia, T. B. Ayed and R. B. Hassen, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2013, 69, o1296 CAS; (b) T. Shibahara, M. Takahashi, A. Maekawa and H. Takagi, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2010, 66, o429 CAS.
- W. Rodriguez-Cordoba, C. Fregoso, E. Jugazagoitia and J. Peon, J. Phys. Chem. A, 2007, 111, 6241 CrossRef CAS PubMed.
- S. R. Stoyanov, J. M. Villegas and D. P. Rillema, Inorg. Chem., 2002, 41, 2941 CrossRef CAS PubMed.
- N. Hamdi, C. Fischmeister, M. C. Puerta and P. Valerga, Med. Chem. Res., 2011, 20, 522 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski, and D. J. Fox, Gaussian 09, Revision D.01, Gaussian, Inc., Wallingford CT, 2009 Search PubMed.
- (a) A. D. Becke, J. Chem. Phys., 1993, 98, 5648 CrossRef CAS PubMed; (b) C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785 CrossRef CAS.
- (a) P. J. Hay and W. R. Wadt, J. Chem. Phys., 1985, 82, 270 CrossRef CAS PubMed; (b) W. R. Wadt and P. J. Hay, J. Chem. Phys., 1985, 82, 284 CrossRef CAS PubMed; (c) P. J. Hay and W. R. Wadt, J. Chem. Phys., 1985, 82, 299 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Association constant determination, detection limit determination, 1H NMR, 13C NMR, ESI MS spectroscopy, UV-vis titration spectra of HL with different metal ions etc. See DOI: 10.1039/c4ra02765e |
| This journal is © The Royal Society of Chemistry 2014 |