Efficient heterogeneous functionalized polymer ionic liquid catalyst for the synthesis of ethylene carbonate via the coupling of carbon dioxide with ethylene oxide†
Bo Zhanga,
Lei Zhanga,
Qinghai Wub,
Quanxi Wangb,
Baoan Songa,
Wenjun Wuc,
Bin Lub and
Tianrui Ren*b
aState Key Laboratory Breeding Base of Green Pesticide and Agricultural Bioengineering/Key Laboratory of Green Pesticide and Agricultural Bioengineering, Ministry of Education, Guizhou University, Guiyang, 550025, P. R. China
bThe Key Laboratory of Resource Chemistry of Ministry of Education, The Development Centre of Plant Germplasm Resources, College of Life and Environmental Science, Shanghai Normal University, 100 Guilin Road, Shanghai, 200234, P. R. China. E-mail: trren@shnu.edu.cn; Fax: +86 21 64328850; Tel: +86 21 64328850
cSchool of Chemistry and Molecular Engineering, East China University of Science and Technology, 130 Meilong Road, Shanghai, 200237, P. R. China
First published on 24th April 2014
Abstract
A series of functionalized polymer ionic liquids (PILs) were synthesized and immobilized onto a 4 Å molecular sieve. The catalytic activity of the resulting heterogeneous catalyst toward the synthesis of ethylene carbonate (EC) via the cycloaddition reaction of ethylene oxide (EO) and CO2 was studied. The effects of the reaction conditions such as reaction temperature, pressure, time, and the amount of catalyst used, were systematically investigated. A high yield of EC and excellent selectivity could be obtained under optimized conditions. The catalyst is thermally stable and shows good reusability. Based on the experimental results, a plausible reaction mechanism has been proposed for the catalytic reaction.
Introduction
Cyclic carbonates, e.g., ethylene carbonate (EC), are valuable chemical products that are used as polar aprotic solvents and electrolytic elements of lithium secondary batteries. Cyclic carbonates can serve as precursors for the formation of polycarbonates as well as intermediates in the production of pharmaceuticals, fine chemicals and agricultural chemicals.1–3 One of the most effective routes for the synthesis of cyclic carbonates is the cycloaddition of CO2 with epoxides.A wide range of homogeneous catalysts such as alkali metal salts,4 organophosphorous compounds,5 organic bases,6 and organometallic compounds7 have been developed, and have exhibited excellent catalytic performance toward the coupling reaction. Unfortunately, those reactions were conducted in homogeneous catalytic systems, which suffer from poor catalyst recovery and product separation. Although heterogeneous catalysts (e.g., metal oxides,8–10 molecular sieves,11–13 and supported catalysts14–16) have been explored, their application is limited because of low activity, and/or poor stability and reusability. Hence, it is crucial to identify more efficient heterogeneous catalysts.
Supported ionic liquids (ILs) are considered promising as efficient heterogeneous catalysts for the synthesis of cyclic carbonates, because they retain important physical and chemical features of ILs, such as nonvolatility, nonflammability, and good thermal stability, as well as high catalytic activity and selectivity. Compared to traditional ILs, the functionalized ionic liquids (e.g., those with hydroxyl or carboxyl groups) were found to show much better catalytic efficiency than single ILs toward the synthesis of cyclic carbonates.17–19 For example, Sun et al. prepared a series of carboxyl IL catalysts containing Brønsted acidic sites and Lewis basic sites, and this type of catalysts showed high catalytic activity with no use of a cocatalyst and organic solvent.20 The immobilization of hydroxyl- or carboxyl-functionalized ionic liquid on a solid support as effective heterogeneous catalysts for the synthesis of cyclic carbonate from the coupling of CO2 with epoxides have exhibited excellent catalytic activity and selectivity.21–24 The excellent catalytic performance can be ascribed to the synergetic effects among OH group (or COOH group) and the halide anions. For example, Han and co-workers23 found that compared with silica-grafted imidazolium-based ILs with hydroxyl groups and with no functional group, carboxylic acid-functionalized imidazolium-based ILs immobilized on silica show the highest activity and selectivity for the synthesis of cyclic carbonates via the cycloaddition of epoxides with CO2, since the –COOH group in the IL cation can greatly accelerate the reactions and showed a synergistic effect with the halide anions. Therefore, the incorporation of carboxylic acid and hydroxyl moieties into ILs could provide an effective alternative for improving the catalytic performance of ILs toward the cycloaddition of CO2 and epoxides.
We synthesized a novel hydroxyl and carboxyl functionalized polymer ionic liquid (PIL) via copolymerization with 1-hydroxyethyl-3-vinyl imidazolium bromide, methyl acrylic acid, hydroxyethyl acrylate and styrene as monomers, and immobilized them onto a 4 Å molecular sieve surface, forming heterogeneous catalysts for the cycloaddition reaction of CO2 and EO. The catalyst is active and selective for the reaction without any use of cocatalyst and organic solvent.
Experimental
Materials
4 Å molecular sieves, styrene, absolute ethanol, dodecyl sodium sulfate (SDS), ammonium persulfate, 1-vinylimidazole, methyl acrylic acid, hydroxyethyl acrylate, sodium hydroxide, 2-bromoethanol, 1-bromoethane, ethylene oxide and ZNX chain extender were purchased from the Sinopharm Chemical Reagent Co., Ltd. CO2 (99.999%) was provided by Successful Gas Industry Co., Ltd. All chemicals were analytic grade and were used without further purification.Preparation of functionalized PIL
The routes to synthesize the functionalized PILs are presented in Scheme 1. Firstly, 1-hydroxyethyl-3-vinyl imidazolium bromide was prepared according to literature.25 1-Vinylimidazole (9.41 g) and absolute ethanol (30 mL) were added to a three-necked flask equipped with a magnetic stirrer. The mixture was heated at 70 °C under a nitrogen atmosphere. A mixture of 2-bromoethanol (12.52 g) and absolute ethanol (20 mL) was dropped very slowly into the flask. The reactants were magnetically stirred for 24 h. Then, the reaction mixture was cooled down, and the unreacted starting materials were removed by distillation in vacuum. After drying under vacuum, 1-hydroxyethyl-3-vinyl imidazolium bromide was obtained. Following a similar procedure, 1-ethyl-3-vinyl imidazolium bromide was also synthesized using 1-bromoethane instead of 2-bromoethanol.The NMR data are as follow: 1-hydroxyethyl-3-vinyl imidazolium bromide: 1H NMR (600 MHz, D2O) δ: 3.84 (m, 2H), 4.26 (m, 2H), 5.32 (dd, 1H), 5.71 (dd, 1H), 7.05 (dd, 1H), 7.49 (s, 1H), 7.68 (s, 1H), 8.97 (s, 1H). 1-Ethyl-3-vinyl imidazolium bromide: 1H NMR (600 MHz, DMSO-d6): 1.48 (t, 3H); 4.25 (q, 2H); 5.42 (dd, 1H); 5.96 (dd, 1H); 7.31 (dd, 1H); 7.98 (s, 1H); 8.24 (s, 1H); 9.61 (s, 1H).
Secondly, the functionalized PILs were synthesized according to the procedures described in Scheme 1. In a typical reaction, methacrylic acid (5.0 g) was neutralized to pH 6 using 0.1 mol L−1 NaOH, and then 1-hydroxyethyl-3-vinyl imidazolium bromide (10 g), hydroxyethyl acrylate (10 g), styrene (1.0 g) were dissolved in the above solution (called solution A). Ammonium persulfate (0.55 g) was dissolved in double-distilled water (10 mL) (called solution B). ZNX chain extender (0.054 g) and double-distilled water (10 mL) were added to a three-necked flask equipped with an electric stirrer, and heated at 80 °C. Solution A and solution B were added into the flask stepwise. Then, the reaction mixture was maintained at 80 °C for 2 h. Subsequently, the reaction mixture was cooled down, the product 2a as a light yellow foam was obtained and dialysed to remove low molecular weight components and other impurities. The obtained foam became solid after freeze-drying. Following a similar procedure, the other functionalized PILs were synthesized.
Synthesis of heterogeneous functionalized PILs
4 Å molecular sieve (10 g), functionalized PIL 2a (5 g) and double-distilled water (20 mL) were added to a three-necked flask under continuous shaking for 24 h at 30 °C followed by vaporization of the water under vacuum. The final product was dried in vacuum at 80 °C for 12 h, and the heterogeneous functionalized PIL 2a (HFPIL2a) was obtained. Following a similar procedure, a series of heterogeneous functionalized PILs (HFPIL1a, HFPIL1b and HFPIL2b) were immobilized onto 4 Å molecular sieve.Cycloaddition reaction
EC was synthesized by a coupling reaction between EO and CO2 in the presence of heterogeneous functionalized PILs. Reaction was performed in a 30 mL stainless-steel reactor with a magnetic stirrer. For a typical catalytic reaction, heterogeneous functionalized PIL (0.5 g) was added to the reactor without any co-solvent. EO (4.4 g) was introduced into the reactor by high-pressure liquid pump. The reactor was placed under a constant pressure of CO2 and then heated to the required temperature. After the appropriate time, the reactor was cooled to room temperature, depressurized and the resulting reaction mixture was separated by filtration. For recycling studies the solid catalyst was washed with absolute ethanol (3 × 10 mL), and dried under vacuum. The products were analyzed on a gas chromatograph (Beifen Co., Ltd. Model, SP-3420) equipped with a capillary column (HP-5, 30 m × 0.32 mm × 0.5 μm) using a flame ionized detector (FID).Characterization
Field emission scanning microscopy (FESEM) was taken with Hitachi S-4800 operating at 1.5 kV. Fourier transform infrared (FT-IR) spectra were collected on a FTIR EQUINO 55 apparatus using KBr pellets. X-ray photoelectron spectroscopy (XPS) spectra were obtained with a Perkin Elmer PHI-5000C ESCA system. The elemental analysis was carried out on a Vario EL III instrument. NMR measurements were conducted on Varian INOVA-400 spectrometer at room temperature. The thermogravimetric analysis was made in a Shimadzu TG-50, at a rate of 10 °C min−1 from ambient temperature to 900 °C.Results and discussion
Characterization of catalyst structure
To confirm the synthesis of the functionalized PIL 2a via copolymerization with 1-hydroxyethyl-3-vinyl imidazolium bromide, methyl acrylic acid, hydroxyethyl acrylate and styrene as monomers, FTIR a spectroscopic study was firstly carried out. The FTIR spectrum of the functionalized PIL (II) is demonstrated in Fig. 1. The functionalized PIL displays a typical strong peak corresponding to –OH stretching frequency centered at about 3380 cm−1. The peaks at 3140, 2950 and 1080 cm−1 are assigned to stretching vibrations of the C–H bonds in the imidazole ring, C–H bonds in alkane, and C–O bonds in monohydric alcohol, respectively. The peak at 1730 cm−1 is related to the carboxyl groups. The characteristic peaks of the imidazole ring and the benzene ring are present at 1570, 1450 and 1400 cm−1. The peaks at 1270 and 1170 cm−1 belong to C–N stretching bands of imidazole ring. The out-of-plane bending vibration absorption peak of monosubstituted benzene derivatives appeared at 750 cm−1. The above result is a clear indication that the functionalized PIL 2a has been synthesized successfully.The chemical compositions of the functionalized PIL 2a have been determined using XPS. The XPS spectrum of the functionalized PIL 2a (Fig. 2) shows the existence of carbon, nitrogen, oxygen, bromine and sodium, illustrating that the functionalized PIL 2a has been successfully synthesized by the four monomers. The above results correlate well with the normal FTIR spectra of the functionalized PIL 2a.
Depicted in Fig. 3 is the TGA curve of the functionalized PIL 2a. It is clear that it is stable up to 250 °C. Upon heating to 700 °C, there is the pyrolysis and complete decomposition of PIL. The results show that the functionalized PIL 2a is thermally stable up to 250 °C.
The morphology and structure of the samples were investigated by FESEM micrograph (Fig. 4). The FESEM images in Fig. 4a and b reveal the morphology of 4 Å molecular sieves. Obviously, 4 Å molecular sieves have a rough surface with porous structure at the surface, suggesting that 4 Å molecular sieves have a relatively large adsorption capacity. The adsorption kinetics studies of functionalized PIL 2a onto 4 Å molecular sieves showed that the absorption equilibrium achieved after 24 h (ESI Fig. S1†), and the adsorption isotherms results obtained at various concentrations showed the adsorption reaches maximum at about 400 mg g−1 when the equilibrium concentration is about 0.6 g mL−1 (ESI Fig. S2†). The functionalized PIL 2a was adsorbed to the surfaces of 4 Å molecular sieves to form so-called heterogeneous functionalized PIL catalyst. In comparison to the original molecular sieves, irregular flakes occurred on the surface of them (Fig. 4c and d), suggesting that the ionic liquid has been successfully immobilized on the support.
 | ||
| Fig. 4 FESEM images of 4 Å molecular sieves (a and b) and heterogeneous functionalized PIL (c and d). | ||
To further prove the presence of the functionalized PIL 2a on 4 Å molecular sieves surface, elemental analysis of the supported PIL catalyst were conducted by XPS (ESI Fig. S3†). The XPS spectrum shows the presence of carbon, nitrogen, oxygen, bromine, silicon and sodium, and the contents of them are 13.17%, 5.66%, 58.85%, 1.69%, 15.75% and 4.88%, respectively.
Catalytic performance
The reaction of EO with CO2 to produce EC was choose as the model reaction to test the activity of the heterogeneous functionalized PILs, and the results are summarized in Table 1. It can be seen that 1-bromoethane, 1-vivylimidazole and 2-bromoethanol are ineffective catalyst for this reaction (Table 1, entries 1–3).| Entry | Catalyst | EO conversion (%) | EC selectivity (%) |
|---|---|---|---|
| a Reaction conditions: EO 0.1 mol, catalyst 0.4 g, initial CO2 pressure 2.5 MPa, time 4 h, temperature 130 °C. | |||
| 1 | 1-Bromoethane | Trace | — |
| 2 | 1-Vinylimidazole | Trace | — |
| 3 | 2-Bromoethanol | Trace | — |
| 4 | HFPIL1a | 40 | 97 |
| 5 | HFPIL1b | 87 | 98 |
| 6 | HFPIL2a | 93 | 97 |
| 7 | HFPIL2b | 54 | 97 |
The activities of HFPIL2a with carboxyl and hydroxyl groups, HFPIL1b with hydroxyl but without carboxyl groups and HFPIL1a in the absence of functional groups were investigated (Table 1, entry 6 vs. 5 and 4). HFPIL2a showed higher activity than HFPIL1b and HFPIL1a. The better catalytic activity of HFPIL2a catalyst is most likely related to the abundant carboxyl and hydroxyl groups, which could provide efficient synergistic sites in accelerating the ring opening of epoxide.17–24 Moreover, the HFPIL2b with carboxyl group but without hydroxyl group (Table 1, entry 7) exhibits higher catalytic activities than HFPIL1a in the absence of functional groups (Table 1, entry 4). It can be seen that the introduction of carboxylic acid moieties into the catalytic system can greatly enhance the catalytic performance of the heterogeneous functionalized PIL catalyst toward the cycloaddition reaction.
Catalytic performance
The catalytic performance of the heterogeneous functionalized PIL catalyst was evaluated for the synthesis of EC from EO and CO2. Fig. 5 depicted the influence of different catalyst–ethylene oxide (EO) mass ratios on cycloaddition reaction of EO and CO2. As shown in Fig. 5, when the amount of catalyst varied from 2% to 9%, conversion rate of EO significantly raised from 73% to 92% and selectivity of EC increased from 94% to 97%. However, the further increasing amount of the catalyst declined EO conversion rate and EC selectivity, which is because that the excess catalyst could not be well dispersed in the reaction mixture and limited the mass transfer between the active sites and reactants.26,27 Consequently, the optimal catalyst amount is about 9%. | ||
| Fig. 5 Effect of catalyst amount on the cycloaddition reaction of EO. Reaction conditions: EO 0.1 mol, initial CO2 pressure 2.5 MPa, time 4 h. | ||
Subsequently, we investigated the effect of some parameters on the catalytic activity of the heterogeneous functionalized PIL catalyst. Almost all the reports on cyclic carbonate synthesis suggest that reaction temperature and CO2 pressure are important parameters affecting the coupling of CO2 and epoxides.23,27,28 Fig. 6 illustrates the dependence of EO conversion rate and EC selectivity on reaction temperature. It was found that the reaction temperature dramatically affected the conversion rate of EO. The EO conversion increases sharply with increasing temperature from 100 °C to 130 °C. The continued increase of temperature beyond 130 °C up to 140 °C increases a little EO conversion. However, further increase in temperature causes a decrease of EO conversion and a slight decrease in EC selectivity, a minute amount of ethylene glycol generated from the hydrolysis of EO is detected as by-product owing to the higher temperature. Such an effect of the higher reaction temperature on catalytic activity has been observed in other catalytic systems.21,27,29 Another reason is that the solubility of the CO2 gas phase in the reaction system decreases with increasing temperature. Therefore, in reactions performed at temperatures greater than 140 °C, CO2 solubility affects reactivity, leading to a decreased in EO conversion. Such an effect of reaction temperature on the synthesis of cyclic carbonates has been observed in the cycloaddition of various epoxides with CO2.28 Therefore, 130 °C is considered suitable for the target reaction.
 | ||
| Fig. 6 Influence of temperature on the catalytic activity Reaction conditions: EO 0.1 mol, catalyst 0.4 g, initial CO2 pressure 2.5 MPa, time 4 h. | ||
Fig. 7 depicts the influence of CO2 pressure on the EO conversion rate and EC selectivity at 140 °C with a reaction time of 4 h. It is observed that PC selectivity is always above 90.0% and can be considered as being independent of CO2 pressure. Interestingly, an increase in CO2 pressure resulted in a moderate increase in EO conversion in the low-pressure region (1.0–2.5 MPa). However, when CO2 pressure is above 2.5 MPa, there is significant decline in EO conversion. Such an effect of CO2 pressure on catalytic activity has been observed in other catalytic systems.5,21,30 For example, when immobilized ionic liquid/ZnCl2 was used for the coupling of PO and CO2, the best catalyst activity appeared at a CO2 pressure of 1.5 MPa at 110 °C.30 A possible explanation is that acidic CO2 dissolves in basic epoxide and liquefies as a result of CO2–epoxide complexing.5,21,30 The high CO2 pressure promotes this kind of CO2–EO interaction rather than enhancing the interaction between EO and catalyst, thus leading to the low catalytic activity. It was also reported that too high CO2 pressure may retard the interaction between the epoxides and the catalyst.20,28,31 Therefore, high CO2 pressure did not benefit the cycloaddition reaction catalyzed by the heterogeneous functionalized PIL catalyst.
 | ||
| Fig. 7 Influence of CO2 pressure on the catalytic activity Reaction conditions: EO 0.1 mol, catalyst 0.4 g, initial CO2 pressure 2.5 MPa, time 4 h, temperature 130 °C. | ||
Additionally, the dependence of EO conversion and EC selectivity on reaction time was also evaluated. It was found that the reaction time had a remarkable influence on the reaction. As shown in Fig. 8, the EO conversion rate was faster in the initial stage and remained almost invariant after 4 h. The reaction time exhibited little influence on the selectivity of EC. Conclusively, a reaction time of 4 h was appropriate for the synthesis of EC in this study.
 | ||
| Fig. 8 Influence of reaction time on the catalytic activity. Reaction conditions: EO 0.1 mol, catalyst 0.4 g, initial CO2 pressure 2.5 MPa, temperature 130 °C. | ||
Experiments were also conducted to examine the recyclability of the heterogeneous functionalized PIL catalyst. In each cycle, the catalyst was recovered by filtration directly. After drying, the catalyst was reused for the next run. As shown in Fig. 9, the catalyst could be reused for six runs, and the EC yield still remained above 90%. Furthermore, it is noteworthy to mention that the EC yield was still remained above 80% after 10 runs, indicating that the catalyst is relatively stable and has excellent catalytic activity. Hence despite the decline after six runs, the catalyst can be considered as reusable. The decline of EC yield after six runs could be due to the functionalized PIL leaching. Poor reusability has been reported before over catalysts of similar kind.22,30
Catalytic activity of the reaction of various epoxides with CO2
Under the optimized conditions the cycloaddition of CO2 to various epoxides using the heterogeneous functionalized PIL was conducted at 130 °C and 2.5 MPa CO2 (Table 2). The results indicate that the catalytic system is applicable to coupling CO2 with a variety of terminal epoxides with high yield and selectivity. Cyclohexene-oxide required a prolonged reaction time compared with the terminal epoxides (Table 2, entry 5) due to steric hindrance.21–24,27,28| Entry | Epoxide | Product | Results | |
|---|---|---|---|---|
| Yield (%) | Selectivity (%) | |||
| a Reaction conditions: epoxide 0.1 mol, catalyst 0.4 g, initial CO2 pressure 2.5 MPa, temperature 130 °C, reaction time 4 h. | ||||
| 1 |  |
 |
93 | 97 |
| 2 | 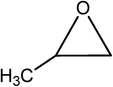 |
 |
90 | 98 |
| 3 |  |
 |
90 | 94 |
| 4 |  |
 |
82 | 97 |
| 5 |  |
 |
31.2 | 97 |
Thermodynamic evaluation of the EC synthesis using EO and CO2 as feedstocks
The thermodynamic phenomena describe how the systems respond to the changes in their surroundings through the effects of heat, work and energy on a system. Therefore, understanding the thermodynamics of EC formation using EO and CO2 as feedstocks is highly important in seeking novel synthesis ideas both in academic investigations and in practical applications. The thermodynamic data of various substances such as ethylene oxide, carbon dioxide and ethylene carbonate are tabulated in Table 3.32,33| Substance | ΔfHmθ (kJ mol−1) | Sfθ (J mol−1 K−1) | ΔfGmθ (kJ mol−1) | Cpm (J mol−1 K−1) |
|---|---|---|---|---|
| CO2 | −393.509 | 213.74 | −394.359 | 26.57 + 42.258 × 10−3T − 14.25 × 10−6T2 |
| EO | −52.63 | 242.53 | −13.01 | 15.21 + 0.1104T |
| EC | −580.88 | 326.69 | −96.127 | −13.165 + 0.3114T − 0.9 × 10−4T2 − 0.3 × 10−7T3 |
The changes of enthalpy, entropy and Gibbs free energy at different temperatures in the process of the synthesis of EC have been calculated from the following formulas respectively32 and the results are given in Table 4.
| ΔrHm = ΣHf(products) − ΣHf(reactants), ΔrSm = ΣSf(products) − ΣSf(reactants) |
| ΔrGm = ΣGf(products) − ΣGf(reactants), ΔrCpm = ΣCpm(products) − ΣCpm(reactants) |
ΔrGT = ΔrHT − TΔrST, ΔrGT = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kp Kp |
| T (K) | ΔrHm (kJ mol−1) | ΔrSm (J mol−1 K−1) | ΔrGm (kJ mol−1) | Kp |
|---|---|---|---|---|
| 298.15 | −239.631 | −311.748 | −146.731 | 5.1 × 1025 |
| 383.15 | −240.575 | −314.580 | −120.091 | 2.393 × 1016 |
| 393.15 | −240.642 | −314.755 | −116.943 | 3.497 × 1015 |
| 403.15 | −241.374 | −314.902 | −114.468 | 6.875 × 1014 |
| 413.15 | −240.750 | −315.025 | −110.645 | 9.873 × 1013 |
It is obvious from Table 4 that the negative of free energy change (ΔrGm) is an indication of spontaneous nature of the EC synthesis from EO and CO2. The negative values of enthalpy change (ΔrHm) for the intervals of temperatures showed the exothermic nature of the reaction. The negative values of ΔrSm, ΔrHm and ΔrGm for the corresponding temperature intervals further suggested low temperature condition is beneficial for the synthesis of EC from EO and CO2, higher temperature is unfavorable for the forward reaction and may induce some side reactions which is consistent with Fig. 6.
The reaction equilibrium constant Kp (Table 4, column 5) within the temperature range from 298.15–413.15 K is very high, indicating that the reaction can not only occur but also be considered as practically irreversible.34 Owing to exothermic reaction, the equilibrium constant Kp decreases with increasing temperature. However, it is noteworthy to mention that the values of Kp remained high in the temperature range of 298.15–413.15 K, and they are thermodynamically advantageous. Therefore, by altering the reaction temperature, the selectivity of the reaction couldn't be improved. Based on the above analysis, it is necessary to study reaction kinetics, thus significantly shortening the reaction time and improving the reaction selectivity and yield, suggesting that it should focus on development of high-activity and high-selectivity catalyst.
The kinetic study of the EC synthesis using EO and CO2 as feedstocks
It has been verified that the synthesis of cyclic carbonate from CO2 and epoxide obey first order kinetics, the reaction are first order in epoxide concentration.35,36 Therefore, dynamic equation of the synthesis of ethylene carbonate from CO2 and EO was established as described in eqn (1). Taking the logarithm of eqn (1) leads to eqn (2).| r = −d[EO]/dt = k[EO] | (1) |
| ln[1/(1 − x)] = kt | (2) |
| T/K | TK−1 × 103 | Fitting equation | R | k/min−1 | ln k |
|---|---|---|---|---|---|
| 383.15 | 2.61 | y = 0.00759x | 0.9993 | 0.00759 | −4.88 |
| 393.15 | 2.54 | y = 0.01339x | 0.9996 | 0.01339 | −4.31 |
| 403.15 | 2.48 | y = 0.01899x | 0.9982 | 0.01899 | −3.96 |
| 413.15 | 2.42 | y = 0.02495x | 0.9998 | 0.02495 | −3.69 |
Reaction activation energy
Fig. 11 is the plots of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k vs. 1/T. Obviously, there is a good linear relations between the logarithm of k and 1/T.
k vs. 1/T. Obviously, there is a good linear relations between the logarithm of k and 1/T.
According to the Arrhenius equation,
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k = ln k0 − Ea/RT k = ln k0 − Ea/RT |
Therefore, the exponential factor k0 and activation energy Ea were obtained as k0 = 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 715.8 and Ea = 51.9965 kJ mol−1. Thus, the kinetic equation of this reaction is r = −dCEO/dt = 98
715.8 and Ea = 51.9965 kJ mol−1. Thus, the kinetic equation of this reaction is r = −dCEO/dt = 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 715.8e−51.9965/RTCEO.
715.8e−51.9965/RTCEO.
Proposed mechanism of coupling reaction
Previous research suggested that Brønsted acids can accelerate the ring-opening reaction of epoxides by forming hydrogen bonds.37 In our current study, functionalized PIL with carboxyl and hydroxyl functional groups exhibits excellent catalytic activity (Table 1 and Fig.5), which is consistent with literature reports.17–24 Based on our results and those of previous reports, the proposed mechanism is shown in Scheme 2. | ||
| Scheme 2 The proposed reaction mechanism for cycloaddition of EO and CO2 catalyzed by heterogeneous functionalized PIL. | ||
The coordination of the H atom of carboxyl and hydroxyl groups of the catalyst with the O atom of EO though hydrogen bonding results in the formation of intermediate. Due to the polarization of the epoxide C–O bonds, the bromide anion (Lewis base) makes a nucleophilic attack on one of the carbon atoms of EO. Simultaneously, there is ring opening of epoxy and generation of an oxy anion. The insertion of CO2 into the haloalkoxy species would result in the formation of a linear halocarbonate that transforms into ethylene carbonate through intramolecular substitution of the halide and regenerates the catalyst. Thus, in our current catalyst system, the coexistence of hydrogen bond donors (–OH and –COOH) and halide anion showed the synergistic effect of promoting the coupling reaction. This effect might be the main contribution to their high catalytic activity and selectivity.
Conclusions
The functionalized PIL with carboxyl and hydroxyl functional groups has been demonstrated as effective catalyst for the synthesis of EC via the cycloaddition of EO with CO2. The functionalized PIL immobilized on the molecular sieve show excellent catalytic activity and selectivity, since the –COOH and –OH groups in the PIL can accelerate the reaction and showed the synergistic effects with the halide anion. Moreover, the heterogeneous catalyst can be easily separated from the products and reused. Based on thermodynamic and kinetic analysis, the reaction is pseudo-first order reaction within the temperature range from 298–413 K.Acknowledgements
The work was financially supported by the National Natural Science Foundation of China (21172147), the National High Technology Research and Development Program of China (863: 2011AA100503), The National Key Technology R & D Program of China (2011BAE06B06-4), National Program on Key Basic Research Project (973: 2010CB126106), the Shanghai Committee of Science and Technology (11ZR1426800).References
- J. M. Sun, S. I. Fujita and M. Arai, Development in the green synthesis of cyclic carbonate from carbon dioxide using ionic liquids, J. Organomet. Chem., 2005, 690, 3490–3497 CrossRef CAS PubMed.
- M. North, R. Pasquale and C. Young, Synthesis of cyclic carbonates from epoxides and CO2, Green Chem., 2010, 12, 1514–1539 RSC.
- Y. B. Xiong, Y. J. Wang, H. Wang, R. M. Wang and Z. P. Cui, Novel one-step synthesis to cross-linked polymeric nanoparticles as highly active and selective catalysts for cycloaddition of CO2 to epoxides, J. Appl. Polym. Sci., 2012, 123, 1486–1493 CrossRef CAS.
- S. R. Jagtap, M. J. Bhanushali, A. G. Panda and B. M. Bhanage, Synthesis of cyclic carbonates from carbon dioxide and epoxides using alkali metal halide supported liquid phase catalyst, Catal. Lett., 2006, 112, 51–55 CrossRef CAS PubMed.
- S. S. Wu, X. W. Zhang, W. L. Dai, S. F. Yin, W. S. Li, Y. Q. Ren and C. T. Au, ZnBr2–Ph4PI as highly efficient catalyst for cyclic carbonates synthesis from terminal epoxides and carbon dioxide, Appl. Catal., A, 2008, 341, 106–111 CrossRef CAS PubMed.
- H. W. Jing and S. T. Nguyen, SnCl4-organic base: Highly efficient catalyst system for coupling reaction of CO2 and epoxides, J. Mol. Catal. A: Chem., 2007, 261, 12–15 CrossRef CAS PubMed.
- F. Jutz, J. D. Grunwaldt and A. Baiker, Mn(III)(salen)-catalyzed synthesis of cyclic organic carbonates from propylene and styrene oxide in “supercritical” CO2, J. Mol. Catal. A: Chem., 2008, 279, 94–103 CrossRef CAS PubMed.
- B. M. Bhanage, S. I. Fujita, Y. Ikushimaa and M. Arai, Synthesis of dimethyl carbonate and glycols from carbon dioxide, epoxides, and methanol using heterogeneous basic metal oxide catalysts with high activity and selectivity, Appl. Catal., A, 2001, 219, 259–266 CrossRef CAS.
- T. Yano, H. Matsui, T. Koike, H. Ishiguro, H. Fujihara, M. Yoshiharaa and T. Maeshima, Magnesium oxide-catalysed reaction of carbon dioxide with an epoxide with retention of stereochemistry, Chem. Commun., 1997, 1129–1130 RSC.
- K. Yamaguchi, K. Ebitani, T. Yoshida, H. Yoshida and K. Kaneda, Mg–Al Mixed Oxides as Highly Active Acid–Base Catalysts for Cycloaddition of Carbon Dioxide to Epoxides, J. Am. Chem. Soc., 1999, 121, 4526–4527 CrossRef CAS.
- E. J. Doskocil, S. V. Bordawekar, B. G. Kaye and R. J. Davis, UV-Vis Spectroscopy of Iodine Adsorbed on Alkali-Metal-Modified Zeolite Catalysts for Addition of Carbon Dioxide to Ethylene Oxide, J. Phys. Chem. B, 1999, 103, 6277–6282 CrossRef CAS.
- M. Tu and R. J. Davis, Cycloaddition of CO2 to Epoxides over Solid Base Catalysts, J. Catal., 2001, 199, 85–91 CrossRef CAS.
- E. J. Doskocil, Effect of Water and Alkali Modifications on ETS-10 for the Cycloaddition of CO2 to Propylene Oxide, J. Phys. Chem. B, 2005, 109, 2315–2320 CrossRef CAS PubMed.
- M. Alvaro, C. Baleizao, D. Das, E. Carbonell and H. Garcia, CO2 fixation using recoverable chromium salen catalysts: use of ionic liquids as cosolvent or high-surface-area silicates as supports, J. Catal., 2004, 228, 254–258 CrossRef CAS PubMed.
- X. H. Zhang, N. Zhao, W. Wei and Y. H. Sun, Chemical fixation of carbon dioxide to propylene carbonate over amine-functionalized silica catalysts, Catal. Today, 2006, 115, 102–106 CrossRef CAS PubMed.
- K. Motokura, S. Itagaki, Y. Iwasawa, A. Miyaji and T. Baba, Silica-supported aminopyridinium halides for catalytic transformations of epoxides to cyclic carbonates under atmospheric pressure of carbon dioxide, Green Chem., 2009, 11, 1876–1880 RSC.
- J. Sun, S. J. Zhang, W. G. Cheng and J. Y. Ren, Hydroxyl functionalized ionic liquid: a novel efficient catalyst for chemical fixation of CO2 to cyclic carbonate, Tetrahedron Lett., 2008, 49, 3588–3591 CrossRef CAS PubMed.
- Y. X. Zhou, S. Q. Hu, X. M. Ma, S. G. Liang, T. Jiang and B. X. Han, Synthesis of cyclic carbonates from carbon dioxide and epoxides over betaine-based catalysts, J. Mol. Catal. A: Chem., 2008, 284, 52–57 CrossRef CAS PubMed.
- L. N. Han, S. J. Choi, M. S. Park, S. M. Lee, Y. J. Kim, M. I. Kim, B. Y. Liu and D. W. Park, Carboxylic acid functionalized imidazolium-based ionic liquids: efficient catalysts for cycloaddition of CO2 and epoxides, React. Kinet., Mech. Catal., 2012, 106, 25–35 CrossRef CAS.
- J. Sun, L. J. Han, W. G. Cheng, J. Q. Wang, X. P. Zhang and S. J. Zhang, Efficient acid–base bifunctional catalysts for the fixation of CO2 with epoxides under metal- and solvent-free conditions, ChemSusChem, 2011, 4, 502–507 CrossRef CAS PubMed.
- W. L. Dai, L. Chen, S. F. Yin, W. H. Li, Y. Y. Zhang, S. L. Luo and C. T. Au, High-Efficiency Synthesis of Cyclic Carbonates from Epoxides and CO2 over Hydroxyl Ionic Liquid Catalyst Grafted onto Cross-Linked Polymer, Catal. Lett., 2010, 137, 74–80 CrossRef CAS PubMed.
- W. L. Dai, L. Chen, S. F. Yin, S. L. Luo and C. T. Au, 3-(2-Hydroxyl-Ethyl)-1-Propylimidazolium Bromide Immobilized on SBA-15 as Efficient Catalyst for the Synthesis of Cyclic Carbonates via the Coupling of Carbon Dioxide with Epoxides, Catal. Lett., 2010, 135, 295–304 CrossRef CAS PubMed.
- L. N. Han, H. J. Choi, S. J. Choi, B. Y. Liu and D. W. Park, Ionic liquids containing carboxyl acid moieties grafted onto silica: Synthesis and application as heterogeneous catalysts for cycloaddition reactions of epoxide and carbon dioxide, Green Chem., 2011, 13, 1023–1028 RSC.
- Y. Y. Zhang, S. F. Yin, S. L. Luo and C. T. Au, Cycloaddition of CO2 to Epoxides Catalyzed by Carboxyl-Functionalized Imidazolium-Based Ionic Liquid Grafted onto Cross-Linked Polymer, Ind. Eng. Chem. Res., 2012, 51, 3951–3957 CrossRef CAS.
- G. Liu, M. Q. Hou, J. Y. Song, T. Jiang, H. L. Fan, Z. F. Zhang and B. X. Han, Immobilization of Pd nanoparticles with functional ionic liquid grafted onto cross-linked polymer for solvent-free Heck reaction, Green Chem., 2010, 12, 65–69 RSC.
- Y. B. Xiong, Z. P. Cui, H. Wang, Y. J. Wang and R. M. Wang, Cycloaddition of CO2 and Epoxides Catalyzed by Polymer-Supported Quaternary Phosphonium Salts, Chin. J. Catal., 2010, 31, 1473–1477 CAS.
- J. Sun, W. G. Cheng, W. Fan, Y. H. Wang, Z. Y. Meng and S. Y. Zhang, Reusable and efficient polymer-supported task-specific ionic liquid catalyst for cycloaddition of epoxide with CO2, Catal. Today, 2009, 148, 361–367 CrossRef CAS PubMed.
- J. Sun, J. Q. Wang, W. G. Cheng, J. X. Zhang, X. H. Li, S. J. Zhang and Y. B. She, Chitosan functionalized ionic liquid as a recyclable biopolymer-supported catalyst for cycloaddition of CO2, Green Chem., 2012, 14, 654–660 RSC.
- M. Ramin, J. D. Grunwaldt and A. Baiker, Behavior of homogeneous and immobilized zinc-based catalysts in cycloaddition of CO2 to propylene oxide, J. Catal., 2005, 234, 256–267 CrossRef CAS PubMed.
- L. F. Xiao, F. W. Li, J. J. Peng and C. G. Xia, Immobilized ionic liquid/zinc chloride: Heterogeneous catalyst for synthesis of cyclic carbonates from carbon dioxide and epoxides, J. Mol. Catal. A: Chem., 2006, 253, 265–269 CrossRef CAS PubMed.
- (a) J. Q. Wang, K. Dong, W. G. Cheng, J. Sun and S. J. Zhang, Insights into quaternary ammonium salts-catalyzed fixation carbon dioxide with epoxides, Catal. Sci. Technol., 2012, 2, 1480–1484 RSC; (b) J. Q. Wang, J. Sun, W. G. Cheng, K. Dong, X. P. Zhang and S. J. Zhang, Experimental and theoretical studies on hydrogen bond-promoted fixation of carbon dioxide and epoxides in cyclic carbonates, Phys. Chem. Chem. Phys., 2012, 14, 11021–11026 RSC.
- X. C. Fu, W. X. Shen, T. Y. Yao and W. H. Hou, Physical Chemistry, 5th edn, Beijing Higher Education Press, 2006, p. 481 Search PubMed.
- R. H. Perry, Perry's Chemical Engineering Hand book, 7th edn, The McGraw-Hill companies, Inc. Industry Press, 1999 Search PubMed.
- S. Y. Ma and D. J. Zhao, Research on equilibrium constant in chemical reaction, Journal of Suihua University, 2005, 25, 152–154 Search PubMed.
- W. Clegg, R. W. Harrington, M. North and R. Pasquale, Cyclic Carbonate Synthesis Catalysed by Bimetallic Aluminium–Salen Complexes, Chem.–Eur. J., 2010, 16, 6828–6843 CrossRef CAS PubMed.
- Y. Li, P. Y. Zhu and Y. R. Wang, Study on the reaction kinetics of ethylene carbonate synthesized by ethylene oxide and carbon dioxide, Huaxue Gongcheng, 2007, 35, 29–32 CAS.
- J. W. Huang and M. Shi, Chemical Fixation of Carbon Dioxide by NaI/PPh3/PhOH, J. Org. Chem., 2003, 68, 6705–6709 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra02689f |
| This journal is © The Royal Society of Chemistry 2014 |








