Au/montmorillonite/polyaniline nanoflakes: facile fabrication by self-assembly and application as catalyst
Youyi Xia*,
Tenjiao Li,
Cong Ma,
Chang Gao and
Jun Chen
Department of Polymer Science and Engineering, School of Chemistry and Chemical Engineering, Anhui University of Technology, Maanshan 243002, P. R. China. E-mail: xiayouyi1027@sohu.com; Fax: +86 555 2311551; Tel: +86 555 2311807
First published on 14th April 2014
Abstract
By the self-assembly between citrate-stabilized Au nanoparticles (NPs) and dedoped montmorillonite (MMT)/polyaniline (PANI) core/shell nanoflakes, Au NP/MMT/PANI nanoflakes could be fabricated facilely. Importantly, hydrogen bonding between Au NPs and the PANI shell of MMT/PANI nanoflakes was proposed to play a pivotal role in driving that self-assembly. The as-prepared product exhibited high catalytic activity when the reduction of p-nitrophenol was taken as a model reaction. These novel findings of the self-assembly mechanism and the preparation process of Au/MMT/PANI nanocatalyst may shed some light on designing and preparing other functional Au NP/conducting polymer composites by self-assembly.
Introduction
Au nanoparticles (NPs) are a fascinating topic due to their unique ability and high catalytic activity, surface enhanced Raman scattering (SERS), etc.1–3 However, it is well known that during their utilization, Au NPs as well as other metallic NPs are easily reduced and apt to form large particles, due to their inherent physical/chemical properties such as autocatalysis, mobility and so on, resulting in poor dispersion and low activity. Loading or embedding Au NPs on/into a support to form nanocomposites is one of the most effective ways to avoid NP aggregation. So far, various materials such as inorganic compounds and polymers4–6 have been often reported as supports. By this method, the migration and aggregation of Au NPs can be avoided, guaranteeing high activity of Au NPs during their practical application.As a good candidate for supporting Au NPs, besides possessing some general advantages, such as high surface area and porous structure, it is more desirable sometimes that electron transfer occurs through a direct or mediated mechanism between the support and Au NPs, which has been proved to effectively enhance the activity of Au NPs.7 For this purpose, some electrical materials or semiconductors8–10 have been selected as substrates for Au NPs. Among them, conducting polymers, especially polyaniline (PANI), are becoming a promising matrix for loading Au NPs to produce novel functional materials due to high conductivity and low cost. The introduction of Au NPs into PANI can be realized by many methods. For example, Chattopadhyay et al. reported a method for synthesizing Au–PANI composites based on the use of H2O2 as the bifunctional reagent for the reduction of HAuCl4 and oxidation of aniline.11 Mallick and Feng et al. found that HAuCl4 could be applied as an oxidant for the polymerization of aniline to produce nanoflakes.12,13 By the reduction of PNAI nanofibers, Han et al. prepared PNAI/Au composite nanofibers with high catalytic activity.14 We also reported using sulfonated PANI-modified silk fibroin fibers to synthesize and load a high density of Au NPs.15 Those one-step methods are very convenient, but the control of both the size distribution and dispersion of Au NPs is not easy even with optimized experimental conditions. Pillalamarri et al.16 reported the synthesis of a Au–PANI composite by using γ-irradiation, in which highly uniform and well-dispersed Au NPs were found on PANI nanofibers. However, the method was expensive and not adapted to produce the composite in bulk.
Recently, Yang's group17 described a novel approach for generating a Au NP/PANI composite with a high density and uniform hot spot structure for SERS only by the self-assembly between citrate-stabilized Au NPs and HCl-doped PANI nanofibers. This method is simple and the size of Au NPs on the composite can be controlled by adjusting the Au NP precursor. It was suggested that the formation was mainly ascribed to the electrostatic attraction between positively charged PANI and negatively charged Au NPs.
Herein, Au/MMT/PANI nanoflakes were synthesized by using a self-assembly method driven by hydrogen bonding. As an example, we investigated the catalytic activity by taking the reduction of p-nitrophenol as a model reaction. The novel findings of the self-assembly mechanism and the preparation process may shed some light on designing and preparing other Au NP/conducting polymer composites.
2. Experimental
2.1. Materials
Aniline (ANI) monomer was purchased from Aldrich and distilled under reduced pressure. HAuCl4, (NH4)2S2O8 (APS), p-nitrophenol (p-NP) and NaBH4 were bought from Chinese Beijing Chem. Co. in their reagent grades and used without further purification. 2-Acrylamido-2-methylpropanesulfonic acid (AMPS) was kindly supplied by All-plus Chemical Co., Ltd (China). Pristine Na+-MMT clay (PGW, cationic (Na+) exchange capacity of 145 meq per 100 g) was purchased from Nanocor, Inc. (USA). The polymerizable tertiary amine (GMA-DEA) used for clay surface modification was synthesized as described elsewhere18 and possessed the following chemical structure.2.2. Surface modification of Na+-MMT
Na+-MMT was modified according to our previous report.19 Typically, 15 g of Na+-MMT clay was dispersed in 450 mL distilled water by stirring overnight at room temperature, into which 50 mL aqueous solution containing 6.45 g of GMA-DEA was added. The pH value of the mixture was adjusted to 3 by using hydrochloric acid. After being stirred for 6 h, the modified MMT clay (GMA-DEA-MMT) was filtered, washed and then redispersed in 500 mL distilled water.Later, 3.0 g AMPS was then added into 150 mL 2 wt% GMA-DEA-MMT dispersion. After the mixture was stirred for 30 min, the initiator (0.03 g APS) was finally introduced in the reactor and the polymerization was allowed to proceed at 70 °C for 4 h under a slow stream of nitrogen. The resulting product was centrifuged and washed repeatedly with distilled water to remove the ungrafted poly(AMPS), and then the novel modified MMT clay (PAMPS-g-MMT) was obtained.
2.3. Preparation of dedoped MMT/PANI nanoflake
PAMPS-g-MMT (5 g) was dispersed in 80 mL of 0.5 mol L−1 hydrochloric acid, into which 0.8 g of ANI was added. After 30 min of stirring, polymerization was started by adding APS (1.96 g). The reaction was allowed to run overnight to ensure completion of polymerization. The resultant precipitate was filtered and sequentially washed with alcohol until the filtrate was clear. Thus, the product, HCl-doped MMT/PANI nanoflakes, could be obtained after being dried at 50 °C in an oven. The dedoped product was obtained by the treatment of HCl-doped MMT/PANI nanoflakes with NH3·H2O (5 wt%), followed by filtering, washing and drying.2.4. Fabrication of Au/MMT/PANI nanoflakes by self-assembly
Citrate-stabilized Au NP solution was first synthesized based on ref. 20. Typically, 2 mL of sodium citrate solution (1 wt%) was added to a stirred HAuCl4 boiling solution (250 mL, 0.001 wt%). After about 75 s, a red solution of citrate-stabilized Au NPs with a diameter of ∼15 nm was collected.Then 2 mg of dedoped PANI/MMT nanoflakes was added to Au NP solution (250 mL), and the mixture was stirred for 24 h or so to get adsorption equilibrium. Then, Au/MMT/PANI nanoflakes could be obtained by being filtered, washed using distilled water and dried at 50 °C in an oven.
2.5. Catalytic activity of Au/PANI/MMT nanoflakes
To study the catalytic activity, 1 mg of Au/PANI/MMT nanoflakes was dispersed in 100 mL p-NP aqueous solution (0.09 mM) at room temperature. Then, 10 mL of freshly NaBH4 aqueous solution (1 M) was added. The progress of the reaction was monitored via UV-Vis spectroscopy by recording the time dependent absorption spectra at a regular time interval of 2 min at room temperature.2.6. Instruments and measurements
The morphology observations and EDS spectroscopy of samples were carried out on a scanning electron microscope (JSM-5610). UV-vis spectra of samples were recorded on a UV-240 spectrometer (Shimadzu, Japan). Zeta potential measurement was carried out on a zeta analyser (Malvern Zetasizer Nano ZS90). The thermal properties were measured on a NETZSCH STA 449F3 thermal analyzer at a heating rate of 10 °C min−1 from 0 to 800 °C. The nitrogen gas flow rate was 20 mL min−1.3. Results and discussion
3.1. Morphology and structure of Au/PANI/MMT nanoflakes
Au/MMT/PANI nanoflakes were synthesized by the self-assembly between citrate-stabilized Au NPs and dedoped core/shell nanoflakes of MMT/PANI, which were prepared via an in situ polymerization of ANI after the modification of Na+-MMT clay. As seen from Fig. 1, this product has a typical flake-like morphology and is decorated with Au NPs, which can be verified by the EDS spectrum (inset of Fig. 1d) (C, N, O, S and Cl coming from PANI). Those Au NPs do not aggregate together and display good dispersion on the as-prepared nanoflakes. | ||
| Fig. 1 SEM images of dedoped MMT/PANI nanoflakes (a) and Au/MMT/PANI nanoflakes prepared by using ∼15 nm Au NPs (b–d). | ||
3.2. Formation process of Au/PANI/MMT nanoflakes
Under the experimental conditions, why and how to form Au/MMT/PANI nanoflakes is worth investigating. Obviously, the formation process should not be ascribed to the electrostatic attraction because both dedoped PANI/MMT nanoflakes (ζ = −1.0 mV) and citrate-stabilized Au NPs are negatively charged. In this case, it is proposed that hydrogen bonding interaction between Au NPs and the outside PANI shell of dedoped PANI/MMT drives the self-assembly process. A schematic demonstration is provided in Fig. 2, and the explanation is given as follows: Au NPs are surrounded by citrate molecules, which provide many oxygen atoms, and the PANI macromolecule contains lots of hydrogen atoms. This results in strong interaction by hydrogen bonding between Au NPs and PANI backbones, which is enough to complete the self-assembly and promote absorption of Au NPs on the PANI shell, although single hydrogen bonding is weak.To test the above proposal, we subsequently chose pure dedoped PANI to replace dedoped PANI/MMT nanoflakes. As expected, a Au/PANI composite can be also obtained. Au NPs display good dispersion on the pure dedoped PANI comparable to that of the as-prepared nanoflakes (Fig. 3).
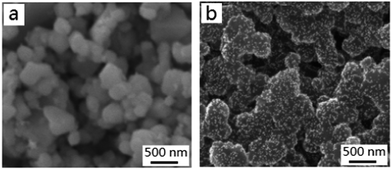 | ||
| Fig. 3 The SEM images of doped PANI (a) and Au/dedoped PANI nanocomposites (b) prepared by the self-assembly between dedoped PANI and Au NPs (∼15 nm). | ||
The thermal properties of Au/PANI/MMT nanoflakes were measured, which can further help to understand the formation mechanism. As shown in Fig. 4, the TG curve of dedoped MMT/PANI nanoflakes is normal. The process of weight loss can be explained according to the decomposition and carbonization of the PANI shell on the MMT/PANI, which has been reported elsewhere.21 As to Au/MMT/PANI nanoflakes, the rate of weight loss is quite different. Before 466 °C, it is higher than that of dedoped MMT/PANI, while the opposite phenomenon is observed after 466 °C. This can be ascribed to the existence of lots of citrate groups in Au/MMT/PANI nanoflakes, which result from the preparation process. Owing to the dual negative charge of dedoped MMT/PANI and citrate groups, hydrogen bonding interaction among –COO− of citrate groups and –NH– of MMT/PANI might be the only reasonable explanation. The higher content of citrate groups causes rapid weight loss before 466 °C due to the gradual water loss and decomposition of sodium citrate (m.p.: 230 °C), which may mainly happen around from 200 °C to 400 °C marked as green in Fig. 3. After 466 °C, the low rate of weight loss can be mainly attributed to Au NPs on the surface of Au/MMT/PANI nanoflakes. This result strongly supports that hydrogen bonding interaction is strong enough to anchor citrate-stabilized Au NPs on the dedoped PANI shell of MMT/PANI.
 | ||
| Fig. 4 TGA patterns of dedoped MMT/PANI nanoflakes and Au/MMT/PANI nanoflakes prepared by using ∼15 nm Au NPs. | ||
3.3. Catalytic activity of Au/MMT/PANI composite nanoflakes
Some advantages such as small size and good dispersion of Au NPs on the as-prepared nanoflakes would make the composites find potential application in the field of catalysis. To investigate the catalytic activity of the nanoflakes, the model catalytic reduction reaction of p-NP by NaBH4 has been performed. This reaction is easily monitored by the decrease in absorption of p-NP at 400 nm.22 From Fig. 5a, it can be found that the absorption of p-NP decreases obviously within 35 min after adding Au/MMT/PANI nanoflakes. Based on this result and no catalytic ability of dedoped MMT/PANI nanoflakes, the reaction kinetics are further discussed (Fig. 5b and c). Obviously, the linear relation of A (A is the absorbance at 400 nm) versus time is observed for the catalyst, indicating that the reaction is better fitted by first-order kinetics.23 The rate constant (Kapp = 1.32 × 10−3 s−1) has been estimated from the slope of the straight line and the initial concentration of p-NP. To further evaluate our experimental result, the activity factor k (the ratio of rate constant Kapp to the total weight of the catalyst W) was calculated to be about 6.60 s−1 g−1 according to ref. 24, indicating a high catalytic activity comparable to some research results.25,26It is well known that reusability is one of the best advantages of using a heterogeneous catalyst rather than a homogeneous catalyst. As a result, the reusability of Au/MMT/PANI nanoflakes as a catalyst toward the reduction of p-NP in the presence of NaBH4 is also discussed. By simple centrifugation and washing after completion of the first cycle, the catalyst can be recovered and reused in the next cycle. As shown in Table 1, the experimental results confirm that Au/MMT/PANI nanoflakes can be used as a recycled catalyst although the catalytic reaction time increases slightly with the number of cycles, which may result from the loss of catalyst during centrifugation and purification processes of catalyst.
| Number of cycles | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Activity factor k (s−1 g−1) | 6.60 | 5.68 | 5.35 | 5.20 | 5.09 |
4. Conclusions
In summary, Au/MMT/PANI nanoflakes have been fabricated by hydrogen bonding-induced self-assembly between negative dedoped MMT/PANI nanoflakes and citrate-stabilized Au NPs. The as-prepared product displays high catalytic activity and good reusability when the reduction of p-nitrophenol is taken as a model reaction. These novel insights of the self-assembly mechanism and the preparation process of Au/MMT/PANI nanocatalysts may shed some light on designing and preparing other functional Au NP-based composite materials for many potential applications such as sensors and a surface-enhanced Raman scattering (SERS) substrate.Acknowledgements
The authors are grateful for financial support of the Anhui Provincial Natural Science Foundation (10040606Q08), Natural Science Foundation of Anhui Education committee (KJ2012A043), National Natural Science Foundation of China (Grant no. 21207001), and Student Research Training Project (SRTP) Foundation of Anhui University of Technology (2013029Y).References
- A. Corma, A. Leyva-Pérez and M. J. Sabater, Gold-catalyzed carbon–heteroatom bond-forming Reactions, Chem. Rev., 2011, 111, 1657–1712 CrossRef CAS PubMed.
- K. Q. Sun, Y. C. Hong, G. R. Zhang and B. Q. Xu, Synergy between Pt and Au in Pt-on-Au nanostructures for chemoselective hydrogenation catalysis, ACS Catal., 2011, 1, 1336–1346 CrossRef CAS.
- J. Zeng, Q. Zhang, J. Y. Chen and Y. N. Xia, A comparison study of the catalytic properties of Au-based nanocages, nanoboxes, and nanoparticles, Nano Lett., 2010, 10, 30–35 CrossRef CAS PubMed.
- S. Goergen, C. Yin, M. Yang, B. Lee, S. Lee, C. Wang, P. Wu, M. B. Boucher, G. Kwon, S. Seifert, R. E. Winans, S. Vajda and M. Flytzani-Stephanopoulos, Structure sensitivity of oxidative dehydrogenation of cyclohexane over FeOx and Au/Fe3O4 nanocrystals, ACS Catal., 2013, 3, 529–539 CrossRef CAS.
- J. Lee, J. C. Park and H. J. A. Song, Nanoreactor framework of a Au@SiO2 yolk/shell structure for catalytic reduction of p-nitrophenol, Adv. Mater., 2008, 20, 1523–1528 CrossRef CAS.
- B. Li, C. Y. Ni and C. Y. Li, Poly(ethylene oxide) Single crystals as templates for Au nanoparticle patterning and asymmetrical functionalization, Macromolecules, 2008, 41, 149–155 CrossRef CAS.
- D. N. Muraviev, J. Macanas, M. Farre, M. Munoz and S. Alegret, Novel routes for inter-matrix synthesis and characterization of polymer stabilized metal nanoparticles for molecular recognition devices, Sens. Actuators, B, 2006, 118, 408–412 CrossRef CAS PubMed.
- W. F. Yan, S. M. Mahurin, Z. W. Pan, S. H. Overbury and S. Dai, Ultrastable Au nanocatalyst supported on surface-modified TiO2 nanocrystals, J. Am. Chem. Soc., 2005, 127, 10480–10481 CrossRef CAS PubMed.
- H. Y. Kim, H. M. Lee and G. Henkelman, CO oxidation mechanism on CeO2-supported Au nanoparticles, J. Am. Chem. Soc., 2012, 134, 1560–1570 CrossRef CAS PubMed.
- S. T. Kochuveedu, Y. H. Jang and D. H. Kim, A study on the mechanism for the interaction of light with noble metal-metal oxide semiconductor nanostructures for various photophysical applications, Chem. Soc. Rev., 2013, 42, 8467–8493 RSC.
- T. K. Sarma, D. Chowdhury, A. Paul and A. Chattopadhyay, Synthesis of Au nanoparticle–conductive polyaniline composite using H2O2 as oxidising as well as reducing agent, Chem. Commun., 2002, 1048–1051 RSC.
- X. Feng, C. Mao, G. Yang, W. Hou and J. J. Zhu, Polyaniline/Au composite hollow spheres: synthesis, characterization, and application to the detection of dopamine, Langmuir, 2006, 22, 4384–4389 CrossRef CAS PubMed.
- K. Mallick, M. J. Witcomb and M. S. Scurrell, Gold in polyaniline: recent trends, Gold Bull., 2006, 39, 4–9 CrossRef.
- J. Han, L. Y. Li and R. Guo, Novel approach to controllable synthesis of gold nanoparticles supported on polyaniline nanofibers, Macromolecules, 2010, 43, 10636–10644 CrossRef CAS.
- Y. Y. Xia, J. M. Wan and Q. F. Gu, Silk fibroin fibers supported with high density of gold nanoparticles: fabrication and application as catalyst, Gold Bull., 2011, 44, 171–176 CrossRef CAS PubMed.
- S. K. Pillalamarri, F. D. Blum, A. T. Tokuhiro and M. F. Bertino, One-pot synthesis of polyaniline–metal nanocomposites, Chem. Mater., 2005, 17, 5941–5946 CrossRef CAS.
- K. Qian, H. L. Liu, L. B. Yang and J. H. Liu, Designing and fabricating of surface-enhanced Raman scattering substrate with high density hot spots by polyaniline template-assisted self-assembly, Nanoscale, 2012, 4, 6449–6454 RSC.
- M. A. Abd El-Ghaffar, N. R. El-Halawany and S. A. Ahmed, Synthesis of glycidyl methacrylate containing diethanol amine and its binary copolymers with ethyl methacrylate and butyl methacrylate as nano-size chelating resins for removal of heavy metal ions, J. Appl. Polym. Sci., 2010, 115, 3063–3073 CrossRef CAS.
- J. Chen, X. Q. Hong, Y. T. Zhao, Y. Y. Xia, D. K. Li and Q. F. Zhang, Preparation of flake-like polyaniline/montmorillonite nanocomposites and their application for removal of Cr(VI) ions in aqueous solution, J. Mater. Sci., 2013, 48, 7708–7717 CrossRef CAS.
- G. Frens, Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions, Nature, 1973, 241, 20–22 CAS.
- P. K. Khanna, N. Singh, S. Charan and A. K. Viswanath, Synthesis of Ag/polyaniline nanocomposite via an in situ photo-redox mechanism, Mater. Chem. Phys., 2005, 92, 214–219 CrossRef CAS PubMed.
- M. H. Rashid and T. K. Mandal, Templateless synthesis of polygonal gold nanoparticles: an unsupported and reusable catalyst with superior activity, Adv. Funct. Mater., 2008, 18, 2261–2271 CrossRef CAS.
- T. Huang, F. Meng and L. Qi, Facile synthesis and one-dimensional assembly of cyclodextrin-capped gold nanoparticles and their applications in catalysis and surface-enhanced Raman scattering, J. Phys. Chem. C, 2009, 113, 13636–13642 CAS.
- J. Li, C. Y. Liu and Y. Liu, Au/graphene hydrogel: synthesis, characterization and its use for catalytic reduction of 4-nitrophenol, J. Mater. Chem., 2012, 22, 8426–8431 RSC.
- K. Kuroda, T. Ishida and M. Haruta, Reduction of 4-nitrophenol to 4-aminophenol over Au NPs deposited on PMMA, J. Mol. Catal. A: Chem., 2009, 298, 7–11 CrossRef CAS PubMed.
- H. Mdrashid, R. R. Bhattacharjee, A. Kotal and T. K. Mandal, Synthesis of spongy gold nanocrystals with pronounced catalytic activities, Langmuir, 2006, 22, 7141–7143 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2014 |




![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)