Non-covalent modification of thrombolytic agent nattokinase: simultaneous improvement of fibrinolysis activity and enzymatic stability†
Abstract
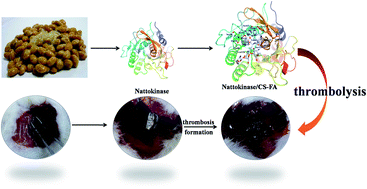
Cardiovascular disease (CVD) has become the leading cause of death globally. Nattokinase (NK), as a novel thrombolytic agent, has gained more and more attention. However, NK is susceptible to chemical oxidation, and subsequent inactivation denaturation. Modification of an enzyme to improve its stability usually brings about falling enzymatic activity. In this study, folic acid modified chitosan (CS-FA) was synthesized, and a series of NK/CS-FA complexes with different mass ratios were prepared. In vitro thrombolysis experiments indicated that NK/CS-FA showed obvious advantages in its fibrinolysis activity and stability. With the NK : CS-FA mass ratio of 100 : 1 (molar ratio of NK : chitosan pyranose ring = 3 : 5), NK/CS-FA 100 : 1 exhibited the best fibrinolysis activity and the thrombi dissolved completely within 17 hours at a constant rate, while the un-modified NK dissolved only 35 wt% of the thrombus in the same duration. NK/CS-FA 100 : 1 retained more than 53% enzymatic activity at 80 °C or pH 2, as a comparison, only 17% enzymatic activity was left for un-modified NK under the same conditions.

 Please wait while we load your content...
Please wait while we load your content...