Improved cyclability of a lithium–sulfur battery using POP–Sulfur composite materials†
Wei Weng‡
a,
Shengwen Yuan‡a,
Nasim Azimiad,
Zhang Jiangb,
Yuzi Liuc,
Yang Renc,
Ali Abouimranea and
Zhengcheng Zhang*a
aChemical Sciences and Engineering Division, Argonne National Laboratory, 9700 South Cass Avenue, Argonne, IL 60439-4837, USA. E-mail: weng@anl.gov; zzhang@anl.gov; syuan@anl.gov; abouimrane@anl.gov; Fax: +1-630-972-4440; Tel: +1-630-252-5258 Tel: +1-630-252-7506 Tel: +1-630-252-3729 Tel: +1-630-252-7868
bAdvanced Photon Source, Argonne National Laboratory, Argonne, IL 60439, USA. E-mail: zjiang@aps.anl.gov; Tel: +1-630-252-3118
cCenter for Nanoscale Materials, Argonne National Laboratory, Argonne, IL 60439, USA. E-mail: yuziliu@anl.gov; ren@aps.anl.gov; Tel: +1-630-252-1775 Tel: +1-630-252-0363
dDepartments of Chemical Engineering and Bioengineering, University of Illinois at Chicago, Chicago, IL 60607, USA. E-mail: nzazimi@anl.gov; Fax: +1-630-972-4440; Tel: +1-630-252-5486
First published on 13th June 2014
Abstract
The microporous nature of the porous organic polymer (POP) successfully limits the crystallization of sulfur and hence restrains the dissolution and diffusion of lithium polysulfides formed during the repeated charge and discharge process of lithium–sulfur batteries. In this study, we demonstrated for the first time that a POP–sulfur nanocomposite can lead to high coulombic efficiency and superior reversibility in lithium–sulfur batteries.
Lithium batteries are one of the most promising energy sources to power the next-generation electric vehicles.1,2 The energy density requirement for a 40-mile all-electric range in a plug-in hybrid electric vehicle (PHEV) is 3–5 times more than that achievable by current lithium-ion technology.3 Thus, development of new, emerging electrode materials with higher energy density is urgently needed for lithium ion batteries. Alternatively, rechargeable batteries beyond lithium-ion have been widely investigated.4 Among them, lithium–sulfur (Li–S) battery is a promising chemistry for a number of desirable properties. Sulfur has high capacity (1675 mA g−1 based on the electrochemical reaction of 16Li + S8 → 8Li2S) and specific energy density (2500 Wh kg−1); sulfur is naturally abundant, environmentally benign and less expensive. Despite the great promise, there are still a number of challenges to be addressed before the practical application, including significant capacity fading during charge–discharge cycling, low coulombic efficiency (CE), insufficient cycle life, and high self-discharge rate.5–9
During the discharge of Li–S battery, a series of lithium polysulfide intermediates (Li2Sx, x = 1–8) are generated. High order lithium polysulfides (4 < x< 8) are generated in the initial discharge stage, which are soluble in the electrolyte and the final discharge products Li2S or Li2S2 are low order polysulfides and tend to precipitate on the surface of the electrode and become unusable. Both the dissolution of polysulfides and the generation of resistive Li2S/Li2S2 lead to the loss of active sulfur material causing fast capacity fade upon cycling.10 During the charge, the dissolved lithium polysulfides are reduced to low order polysulfides on the lithium anode, which then diffuses back to the sulfur cathode and gets oxidized, causing a so-called “redox shuttling” phenomenon significantly lowering the columbic efficiency. Accordingly, many studies have been conducted to solve the issues associated with this battery chemistry. There are reports on Li–S battery using coated current collector,11 coated porous separator for enhanced electrochemical performance of Li–S battery,12 and new electrolyte to improve performance of Li–S battery.13 Most research are aimed at maintaining the mechanical and electrical integrity of the sulfur electrode by embedding lithium polysulfides within the framework of a substrate, such a substrate could be mesoporous carbon,14–17 carbon nanotube,18–20 carbon nanofiber,21 carbon sphere,22 graphene,23–25 graphene oxide26–28 and conductive polymer.29–32 Although great improvement has been achieved, the synthesis of such materials with controlled size and the uniform distribution of the pores poses a great challenge.33 In this communication, we report a new sulfur electrode embedded in an easily available porous organic polymer (POP) network.
POPs represent a new class of amorphous polymeric materials with tuneable permanent porosity.34–36 The polymer networks possess large Brunauer–Emmett–Teller (BET) surface area comparable to that of crystalline inorganic microporous materials, such as zeolites, silicas, activated carbon, and metal–organic frameworks. POPs are constructed exclusively by strong covalent bonds of rigid backbone (struts) and aromatic building blocks (nodes). The high rigidity of the materials is crucial for the mitigation of the chain flexibility, which can reduce the fluctuation of pore size and render the pore structure substantially more stable. The pore geometry and pore size distribution can be controlled by careful selection of 3D-directing “nodes” with “struts” of different lengths. Due to their unique physicochemical property, POPs have found many applications in gas storage, separation, and catalysis. However, little research has been conducted in the electrochemical energy storage system. It is our original idea to investigate the high surface area POP as a substrate to host active sulfur material for Li–S battery. The non-polar groups in the POP have strong interaction with the non-polar sulfur and the conductive additive. POP substrate is expected to restrain the sulfur and the Li2Sx and prevent the dissolution and subsequent diffusion of Li2Sx species into the electrolyte thus enhancing the reversibility of the Li–S battery.
In this communication, we report the improved cyclability of a Li–S battery using POP–sulfur composite material. Fig. 1 showed the chemical structure of three polymers, POP-A, POP-B and POP-C, which can be readily prepared following the previously reported procedure of a one-step polymerization reaction with suitable monomers.37,38 The detailed synthesis of these POPs are described in ESI.† The BET surface area of the porous carbon materials used for sulfur–carbon composite14–22 is usually less than 1000 m2 g−1. In contrast, POPs in this study have much higher surface area (up to 3143 m2 g−1) than the porous carbon materials. Moreover, these POPs exhibit dominant micropores with pore size less than 1 nm as calculated from the Non-Local Density Functional Theory (NLDFT) (Fig. S1 in ESI†). The highly strained tetrahedral monomer in POP-B and POP-C cross-links with three or four other monomers to extend the network three dimensionally thus enables the formation of a robust network with less penetration and high surface area.
Fig. 2a and b show 1D Wide-angle X-ray scattering (WAXS) pattern and the pore size distribution of the POPs fitted to the Percus–Yevick hard-sphere approximation.39–41 Fig. 2c gives the comparison of the pore size measured by the X-ray technique and calculated by NLDFT method. The difference is probably caused by the ideal sphere model used for pore shapes, which ignores the irregular cross-linked molecular architecture. Both X-ray and NLDFT data, however, are qualitatively consistent with each other: POP-B has the largest pore size and POP-A has the smallest pore size.
POP–sulfur composites were prepared by the thermal treatment of the mixture of sulfur and POPs after homogenization by a ball milling process as illustrated in Fig. 3. The melting-down process afforded micro-size agglomerates of POP and sulfur composites in all three samples as observed by SEM (Fig. S2 in ESI†). Transmission electron microscopy (TEM) of the POP-A–sulfur composite is shown in Fig. 4a and b. No diffraction contrast from grains was observed in the POP-A–sulfur images confirming that sulfur particles are well dispersed in the POP matrix. This is in good agreement with the results from WAXS experiment and no sharp diffraction peaks of crystalline sulfur was observed for POP-A–sulfur composite (Fig. 2d). The elemental mapping by energy-filtered TEM further confirms a homogeneous distribution of sulfur in the porous polymer even at the nanoscale, as shown in Fig. 4c–e.
 | ||
| Fig. 3 Preparation of POP–sulfur composites with micropores as host materials for accommodation of nanosized sulfur particles. | ||
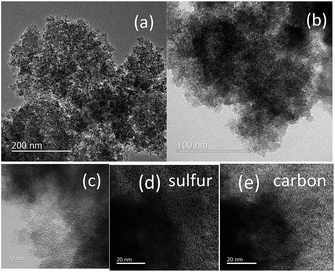 | ||
| Fig. 4 TEM images of POP-A–S composite (a) and (b), TEM image (c) and corresponding EDX elemental mappings of POP-A–S composite for sulfur (d) and carbon (e). | ||
Another evidence of the incorporation of sulfur into the micropores of POPs is the significant reduction of the BET surface area after the ball milling and thermal treatment. The surface area of POP-A is reduced from 1362 m2 g−1 to 104 m2 g−1 when the composite (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by weight) is formed and this reduction is more drastic for POP-B and POP-C. Additionally, the total pore volume of POPs also decreases dramatically after encapsulation with sulfur (in cm3 g−1, POP-A: 1.292; POP-A–sulfur: 0.20; POP-B: 2.07; POP-B–sulfur: 0.09; POP-C: 0.94; POP-C–sulfur: 0.05), suggesting that sulfur is infused into the POP pores.
1 by weight) is formed and this reduction is more drastic for POP-B and POP-C. Additionally, the total pore volume of POPs also decreases dramatically after encapsulation with sulfur (in cm3 g−1, POP-A: 1.292; POP-A–sulfur: 0.20; POP-B: 2.07; POP-B–sulfur: 0.09; POP-C: 0.94; POP-C–sulfur: 0.05), suggesting that sulfur is infused into the POP pores.
The charge–discharge voltage profiles of Li–S cells containing POP–sulfur composite are presented in Fig. 5. Fig. 5a shows the first charge and discharge voltage profiles for POP-A–sulfur cell. The discharge capacity is 927 mA h g−1 at a current density of 200 mA g−1 and the subsequent charge capacity is 898 mA h g−1, corresponding to a high coulombic efficiency of 97%. As shown in Fig. 5b, the high coulombic efficiency for POP-A–sulfur cell is maintained even after 100 cycles, suggesting the successful restraint of polysulfide dissolution and the redox shuttling effect. Such a high coulombic efficiency in the whole cycle range for a sulfur cathode is quite rare.42 Wang et al.43 reported a PAN/sulfur composite cathode maintained a capacity of 600 mA h g−1 after 50 cycles. More recently, a PAN nanotube/sulfur cathode by Liu et al.29 was reported to deliver a capacity of 837 mA h g−1 after 100 cycles at low rate. However, for both composite cathodes, a long activation process (∼20 cycles) is required to reach the maximum discharge capacity, and the shuttle mechanism still exists. The excellent performance of POP-A–sulfur composite clearly indicates that the highly porous structure and high hydrophobic nature of the POPs significantly suppresses the dissolution of polysulfides and enables a highly reversible Li–S battery.
Li–S cell testing data for POP-B–sulfur and POP-C–sulfur composites are shown in Fig. 5c and d. It is notable that the charge and discharge voltage profiles of POP–sulfur composites showed different potential polarization, which might be caused by the different porous structure and the pore volume reduction after the sulfur infusion. As shown in Fig. 5d, both POP-B and POP-C composite cells can achieve high coulombic efficiency in the initial cycle and the values are slightly greater than 100% in the following cycles, indicating that the shuttle mechanism was completely suppressed in the POP-B and POP-C systems. The reversibility of the three POP–sulfur composites is impressive given the absence of the widely reported efficient additives such as LiNO3,44 P2S5,45 fluorinated ether13 or lithium polysulfide dissolved in the electrolyte as electro-active catholyte.46,47
In conclusion, the porous organic polymer with microporous structure and large surface area has been demonstrated for the first time as an effective substrate to accommodate sulfur active materials and suppress the lithium polysulfides dissolution. The POP–sulfur nanocomposite electrodes showed excellent reversibility in Li–S cell. This research provides an insight that POPs play a vital role in addressing the technical barriers for the lithium–sulfur chemistry. However, for the practical use in electric vehicles, the high practical energy density for Li–S battery is extremely desired. For the future work, POPs could be replaced by a conductive polymer with unique microporous structure such as conjugated microporous polymers (CMPs) that combine π-conjugated skeletons with permanent nanopores with the hope to eliminate or reduce the amount of conducting agent in the sulfur electrode to greatly improve the practical energy density of Li–S battery.46
Acknowledgements
This work was supported by the Center for Electrical Energy Storage: Tailored Interfaces, an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences. Electron Microscopy center for Materials Research, Center for Nanoscale Materials and Advanced Photon Source are also acknowledged. Argonne, a U.S. Department of Energy Office of Science laboratory, is operated under Contract no. DE-AC02-06CH11357.Notes and references
- M. M. Thackeray, C. Wolverton and E. D. Isaacs, Energy Environ. Sci., 2012, 5, 7854 CAS.
- M. Armand and J. M. Tarascon, Nature, 2008, 451, 652 CrossRef CAS PubMed.
- V. Etacheri, R. Marom, R. Elazari, G. Salitra and D. Aurbach, Energy Environ. Sci., 2011, 4, 3243 CAS.
- (a) Z. Peng, S. A. Freunberger, Y. Chen and P. G. Bruce, Science, 2012, 337, 563 CrossRef CAS PubMed; (b) H.-G. Jung, J. Hassoun, J.-B. Park, Y.-K. Sun and B. Scrosati, Nat. Chem., 2012, 4, 579 CrossRef CAS PubMed; (c) J. Muldoon, C. B. Bucur, A. G. Oliver, T. Sugimoto, M. Matsui, H. S. Kim, G. D. Allred, J. Zajicek and Y. Kotani, Energy Environ. Sci., 2012, 5, 5941 RSC; (d) T. Zhang, Z. Tao and J. Chen, Mater. Horiz., 2014, 1, 196 RSC.
- H. Yamin and E. Peled, J. Power Sources, 1983, 9, 281 CrossRef CAS.
- Y. Fu and A. Manthiram, RSC Adv., 2012, 2, 5927 RSC.
- X. L. Ji, K. T. Lee and L. F. Nazar, Nat. Mater., 2009, 8, 500 CrossRef CAS PubMed.
- J. Shim, K. A. Striebel and E. J. Cairns, J. Electrochem. Soc., 2002, 149, A1321 CrossRef CAS PubMed.
- H. Yamin, A. Gorenshtein, J. Penciner, Y. Sternberg and E. Peled, J. Electrochem. Soc., 1988, 135, 1045 CrossRef CAS PubMed.
- K. Kumaresan, Y. Mikhaylik and R. E. White, J. Electrochem. Soc., 2008, 155, A576 CrossRef CAS PubMed.
- L. Wang, X. He, J. Li, J. Gao, M. Fang, G. Tian, J. Wang and S. Fan, J. Power Sources, 2013, 239, 623 CrossRef CAS PubMed.
- Z. Zhang, Y. Lai, Z. Zhang, K. Zhang and J. Li, Electrochim. Acta, 2014, 129, 55 CrossRef CAS PubMed.
- N. Azimi, W. Weng, C. Takoudis and Z. Zhang, Electrochem. Commun., 2013, 37, 96 CrossRef CAS PubMed.
- X. L. Li, Y. L. Cao, W. Qi, L. V. Saraf, J. Xiao, Z. M. Nie, J. Mietek, J. G. Zhang, B. Schwenzer and J. Liu, J. Mater. Chem., 2011, 21, 16603 RSC.
- J. Schuster, G. He, B. Mandlmeier, T. Yim, K. T. Lee, T. Bein and L. F. Nazar, Angew. Chem., Int. Ed., 2012, 51, 3591 CrossRef CAS PubMed.
- S. R. Chen, Y. P. Zhai, G. L. Xu, Y. X. Jiang, D. Y. Zhao, J. T. Li, L. Huang and S. G. Sun, Electrochim. Acta, 2011, 56, 9549 CrossRef CAS PubMed.
- Y. Yang, G. H. Yu, J. J. Cha, H. Wu, M. Vosgueritchian, Y. Yao, Z. A. Bao and Y. Cui, ACS Nano, 2011, 5, 9187 CrossRef CAS PubMed.
- S. Dorfler, M. Hagen, H. Althues, J. Tubke, S. Kaskel and M. J. Hoffmann, Chem. Commun., 2012, 48, 4097 RSC.
- J. C. Guo, Y. H. Xu and C. S. Wang, Nano Lett., 2011, 11, 4288 CrossRef CAS PubMed.
- S. S. Jeong, Y. Lim, Y. J. Choi, G. B. Cho, K. W. Kim, H. J. Ahn and K. K. Cho, J. Power Sources, 2007, 174, 745 CrossRef CAS PubMed.
- L. Ji, M. Rao, S. Aloni, L. Wang, E. J. Cairns and Y. Zhang, Energy Environ. Sci., 2011, 4, 5053 CAS.
- B. Zhang, X. Qin, G. R. Li and X. P. Gao, Energy Environ. Sci., 2010, 3, 1531 CAS.
- H. L. Wang, Y. Yang, Y. Y. Liang, J. T. Robinson, Y. G. Li, A. Jackson, Y. Cui and H. J. Dai, Nano Lett., 2011, 11, 2644 CrossRef CAS PubMed.
- J. Z. Wang, L. Lu, M. Choucair, J. A. Stride, X. Xu and H. K. Liu, J. Power Sources, 2011, 196, 7030 CrossRef CAS PubMed.
- N. W. Li, M. B. Zheng, H. L. Lu, Z. B. Hu, C. F. Shen, X. F. Chang, G. B. Ji, J. M. Cao and Y. Shi, Chem. Commun., 2012, 48, 4106 RSC.
- F. F. Zhang, X. B. Zhang, Y. H. Dong and L. M. Wang, J. Mater. Chem., 2012, 22, 11452 RSC.
- L. W. Ji, M. M. Rao, H. M. Zheng, L. Zhang, Y. C. Li, W. H. Duan, J. H. Guo, E. J. Cairns and Y. G. Zhang, J. Am. Chem. Soc., 2011, 133, 18522 CrossRef CAS PubMed.
- L. Zhang, L. Ji, P.-A. Glans, Y. Zhang, J. Zhu and J. Guo, Phys. Chem. Chem. Phys., 2012, 14, 13670 RSC.
- L. F. Xiao, Y. L. Cao, J. Xiao, B. Schwenzer, M. H. Engelhard, L. V. Saraf, Z. M. Nie, G. J. Exarhos and J. Liu, Adv. Mater., 2012, 24, 1176 CrossRef CAS PubMed.
- F. Wu, J. Z. Chen, R. J. Chen, S. X. Wu, L. Li, S. Chen and T. Zhao, J. Phys. Chem. C, 2011, 115, 6057 CAS.
- F. Wu, S. X. Wu, R. J. Chen, J. Z. Chen and S. Chen, Electrochem. Solid-State Lett., 2010, 13, A29 CrossRef CAS PubMed.
- J. Wang, J. Chen, K. Konstantinov, L. Zhao, S. H. Ng, G. X. Wang, Z. P. Guo and H. K. Liu, Electrochim. Acta, 2006, 51, 4634 CrossRef CAS PubMed.
- J. Lee, J. Kim and T. Hyeon, Adv. Mater., 2006, 18, 2073 CrossRef CAS.
- P. Kaur, J. T. Hupp and S. T. Nguyen, ACS Catal., 2011, 1, 819 CrossRef CAS.
- Y. Zhang and S. N. Riduan, Chem. Soc. Rev., 2012, 41, 2083 RSC.
- N. B. McKeown and P. M. Budd, Chem. Soc. Rev., 2006, 35, 675 RSC.
- C. D. Wood, B. Tan, A. Trewin, H. Niu, D. Bradshaw, M. J. Rosseinsky, Y. Z. Khimyak, N. L. Campbell, R. Kirk, E. Stöckel and A. I. Cooper, Chem. Mater., 2007, 19, 2034 CrossRef CAS.
- S. Yuan, B. Dorney, D. White, S. Kirklin, P. Zapol, L. Yu and D.-J. Liu, Chem. Commun., 2010, 46, 4547 RSC.
- D. J. Kinning and E. L. Thomas, Macromolecules, 1984, 17, 1712 CrossRef CAS.
- J. J. Pedersen, Appl. Crystallogr., 1994, 27, 595 CrossRef.
- T. Sun, S. Donthu, M. Sprung, K. D'Aquila, Z. Jiang, A. Srivastava, J. Wang and V. P. Dravid, Acta Mater., 2009, 57, 1095 CrossRef CAS PubMed.
- W. Weng, V. G. Pol and K. Amine, Adv. Mater., 2013, 25, 1608 CrossRef CAS PubMed.
- J. Wang, J. Yang, J. Xie and N. Xu, Adv. Mater., 2002, 14, 963 CrossRef CAS.
- D. Aurbach, E. Pollak, R. Elazari, G. Salitra, C. S. Kelley and J. J. Affinito, J. Electrochem. Soc., 2009, 156, A694 CrossRef CAS PubMed.
- Z. Lin, Z. Liu, W. Fu, N. J. Dudney and C. Liang, Adv. Funct. Mater., 2013, 23, 1064 CrossRef CAS.
- R. Xu, I. Belharouak, J. C. M. Li, X. Zhang, I. Bloom and J. Bareño, Adv. Energy Mater., 2013, 3, 833 CrossRef CAS.
- S. Chen, F. Dai, M. L. Gordin and D. Wang, RSC Adv., 2013, 3, 3540 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedure, illustration of coin cell assembly with different prelithiation techniques. See DOI: 10.1039/c4ra02589j |
| ‡ W.W. and S.Y. contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2014 |



