The preferred radical scavenging mechanisms of fisetin and baicalein towards oxygen-centred radicals in polar protic and polar aprotic solvents
Abstract
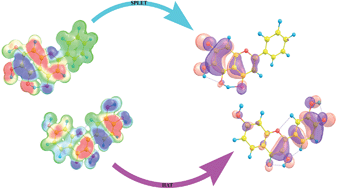
Naturally occurring flavonoid molecules, i.e. fisetin (2-(3,4-dihydroxyphenyl)-3,7-dihydroxychromen-4-one) and baicalein (5,6,7-trihydroxy-2-phenyl-4H-chromen-4-one), have been investigated experimentally and theoretically for their ability to scavenge hydroxyl and superoxide anion radicals. The reaction enthalpies for the reaction of fisetin and baicalein with selected radical species, related to three mechanisms of free radical scavenging activity (HAT, SET-PT and SPLET), are calculated using the M05-2X/6-311+G(d,p) model. The calculated energy requirements indicated the preferred radical scavenging mechanisms in polar protic and aprotic solvents.

 Please wait while we load your content...
Please wait while we load your content...