Co-production of xylanase and xylooligosaccharides from lignocellulosic agricultural wastes
D. Sutay Kocabas*a and
N. Ozbenb
aDepartment of Food Engineering, Karamanoglu Mehmetbey University, Yunus Emre Yerleskesi, 70100-Karaman, Turkey. E-mail: didemkocabas@kmu.edu.tr; Fax: +90 (0) 338 2262214; Tel: +90 (0) 338 2262000/5008
bDepartment of Biology, Karamanoglu Mehmetbey University, 70100, Karaman, Turkey
First published on 27th May 2014
Abstract
Lignocellulosic biomass including agricultural residues and by-products, and woody biomass are produced worldwide in large amounts every year. With the exception of limited-usage areas, these materials are regarded as waste. Therefore, renewable lignocellulosic biomass is gaining considerable attention because of its potential for use in the production of biofuels and other value-added products. In this study, the co-production of two value-added products, xylanase (enzyme) and xylooligosaccharides (XO), was investigated by utilizing lignocellulosic biomass residues within a multi-product biorefinery framework. Box–Behnken design-based response surface methodology was employed to optimize culture parameters for xylanase production from a thermophilic fungus, Scytalidium thermophilum, and the enzyme was biochemically characterized. Optimized media conditions resulted in an approximately 2-fold increase in xylanase activity compared to the initial conditions. Crude enzyme solution was capable of producing XO from the hemicellulosic fraction of various biomass residues. The highest hemicellulose extraction yield was achieved from corn cob (37.7 ± 1.5%), followed by wheat bran (26.6 ± 0.8%) and cotton stalks (22.2 ± 0.6%). The highest reducing end concentration was obtained from sunflower seed shell (4.89 ± 0.02 mg mL−1), followed by sunflower stalks (4.41 ± 0.03 mg mL−1) and corn cob (3.49 ± 0.09 mg mL−1). Accordingly, corn cob yielded the highest XO production (172.1 mg XO per g raw biomass residue) with the main products of xylose (X1) and xylobiose (X2). In terms of the hemicellulosic fraction, beechwood had the maximum XO yield with 793 mg XO per g hemicellulose. Sunflower seed shell hydrolysis yielded mainly X1 and xylotetrose (X4) without X2 and xylotriose (X3). After 1 h of reaction time, X2 and higher sugars were obtained from the commercial beechwood xylan, while only X1 and X4 were obtained after 6 h. Xylose-free xylobiose was obtained from sugar beet bagasse and wheat bran, which are regarded as potential XO sources because XO with degrees of polymerization from 2–4 are preferred in food applications and XO without xylose are important in the food industry from a prebiotic point of view.
1. Introduction
Lignocellulosic biomass, especially inedible crops, are expected to become one of the major renewable energy resources in the near future. The utilization of renewable sources can help to deal with global warming and the depletion of fossil fuel resources. In addition, their use allows the sustainable production of chemicals and fuels without affecting human food supplies.1 As a result of continuous global population growth and decreasing available food resources, hunger problems are arising every day. Therefore, when alternative non-food sources exist, using food resources as industrial raw materials is not ideal. Currently, sugar and its derivatives are widely used as carbon sources in industrial fermentation processes; in recent years, however, the utilization of renewable lignocellulosic biomass as an alternative carbon source has attracted more attention. Agro-industrial wastes such as sugar cane bagasse, wheat bran, rice bran, corn cob and wheat straw are known as the cheapest and most widely accessible natural carbon sources.2 Corn cob is a favourable alternative biomass for fermentation processes because of its renewable nature, wide availability, very low economical value and high hemicellulose content.3,4Hemicellulosic fractions of plant tissues are mainly composed of xylan, which accounts for approximately one-third of all renewable organic carbon on Earth.5 Xylanases are glycosidases (O-glycoside hydrolases, EC 3.2.1.x) that catalyze the hydrolysis (depolymerization) of xylan.6 Xylan possesses a complex and highly-branched heteropolymeric structure. The hydrolysis of xylan is carried out by the xylanolytic enzyme system, in which endo-1,4-β-xylanase and β-xylosidase are the main enzymes.6,7 Xylanases have a variety of applications related to paper and pulp, food, textiles, the pharmaceutical industry and the bioconversion of lignocellulosics to sugar, ethanol and other value-added products. Because of their extensive use, xylanases have become a very important enzyme group from an economic point of view, and xylanase production processes have gained considerable attention from researchers.8,9 Xylanases are produced by a number of microorganisms, mainly bacteria and fungi.5 Microbial xylanases are the preferred catalysts in industry; in particular, filamentous fungi produce extracellular xylanase at higher levels compared to yeasts or bacteria, making fungal xylanases favourable at the industrial scale.10,11
Xylooligosaccharides (XO) are sugar oligomers made up of xylose units and produced by the hydrolysis of xylan.12 XO are non-digestible food ingredients with prebiotic effects; they are neither hydrolysed nor absorbed in the upper part of the gastrointestinal tract and help stimulate the growth of a limited number of health-improving bacteria. XO have favourable technological features such as stability at acidic pH values and high temperatures. They also have a low available energy content, giving them the potential to reduce obesity.13–15 The utilization of lignocellulosic biomass for XO production can be carried out with both chemical and biochemical methods, resulting in a variety of undesired components and large amounts of monosaccharides. XO production by enzymatic hydrolysis is recommended as it avoids unwanted toxic products and the use of specialized equipment.14,16 Different types of agricultural biomass have been investigated for the production of XO, and large-scale XO production is currently performed using lignocellulosic biomass.16 Therefore, to achieve the goal of economically converting hemicellulose (biomass) to value-added products, it is important to identify specific xylanases capable of producing XO from different xylan sources.1 A lignocellulosic biomass was used in this study for two purposes: as the carbon source for xylanase production in the fermentation medium and as the raw material for XO production. A xylan source must be used in both xylanase and XO production; this source can be either an expensive commercial product or a lignocellulosic material, which has very low or no economic value. In fact, commercial xylan is also produced from lignocellulosic starting materials. To make the process cost-effective, waste and cheap sources (lignocellulosics in this case) are to be preferred. The possible requirement of a pre-treatment step when using lignocellulosic biomass for xylan production is one disadvantage of this strategy.
Industrial process optimization studies aimed at increasing product yield and reducing process time and cost generally involve statistical experimental design techniques because one-factor-at-a-time approaches are time-consuming, require several experiments and may produce unreliable results.17 To overcome these drawbacks, response surface methodology (RSM) is usually employed. The aim of this technique is to achieve the best performance or quality characteristics (response) by testing different levels of inputs (independent variables) using the minimum number of experiments.18 In this study, Box–Behnken Design (BBD)-based RSM was used to optimize xylanase production from Scytalidium thermophilum utilizing corn cob waste as carbon source in the fermentation culture. The enzyme was biochemically characterized and used for XO production from various lignocellulosic biomass residues. To the best of our knowledge, previous studies have mainly focused on XO production from various commercial xylanases; studies on the production of XO using xylanases produced from microorganisms are much less common. This study describes the simultaneous production of an enzyme (xylanase) and a high value product (XO) through the utilization of lignocellulosic biomass.
2. Material and methods
2.1. Lignocellulosic material
Tested biomass residues and their producer cities in Turkey were as follows: corn cobs (Adapazari), cotton stalk (Sanliurfa), sunflower stalk and sunflower seed shell (Kutahya) and wheat bran (Eskisehir). Sugar beet bagasse was kindly provided by Konya Seker sugar factory (Konya, Turkey).2.2. Microorganism and culture conditions
Scytalidium thermophilum (Humicola insolens, ATCC no. 16454) was cultivated on YpSs agar plates containing (g L−1): yeast extract (4.0); K2HPO4 (1.0); MgSO4·7H2O (0.5); soluble starch (15.0); and agar (20.0) in distilled water.19 The starting medium (centre point for BBD) used for xylanase production was composed of (g L−1): corn cob as the carbon source (30); yeast extract (4); K2HPO4 (1); MgSO4·7H2O (0.5); and CuSO4·(5H2O (0.1). Medium centre point values were determined according to the study of Ogel et al. on endoglucanase production from S. thermophilum on complex lignocellulosic biomass.20 Corn cobs were ground to a particle size smaller than 2 mm in a laboratory hammer mill. A 24 h-old preculture (2 mL) was used to inoculate the main culture. Fermentation was performed at 45 °C with a 155 rpm shaking rate using 100 mL of medium in 250 mL Erlenmeyer flasks. For experimental sets other than the centre point tests, inoculum level, temperature, pH and carbon (corn cob) and nitrogen (yeast extract) source concentrations were adjusted according to the experimental design. Samples were withdrawn on a daily basis from the fermentation medium, filtered through Whatmann no. 1 filter paper and centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rcf for 10 min. The clear supernatant solution was used as the crude enzyme preparation for further analysis.
000 rcf for 10 min. The clear supernatant solution was used as the crude enzyme preparation for further analysis.
2.3. Assay of xylanase activity
Beechwood xylan solution was prepared at a concentration of 1.0% (w/v) in 50 mM sodium phosphate buffer (pH 7.0) and used as the substrate for the enzyme assay.21 Xylanase activity was determined by measuring the reducing sugar released from beechwood xylan by the 3,5-dinitrosalicylic acid (DNSA) method.22 The reaction mixture contained 10 mL of 1.0% (w/v) beechwood xylan solution and 1 mL of suitable diluted crude enzyme preparation in 50 mM sodium phosphate buffer (pH 7.0). The enzymatic reaction was carried out at 60 °C. Samples (1 mL) were taken from the reaction mixture at defined time intervals, immediately mixed with 1.5 mL of DNSA and boiled for 5 min. Sample colour development was determined spectrophotometrically at a wavelength of 540 nm. One unit of xylanase activity (IU) is defined as the amount of enzyme that liberates 1 μmol of xylose per minute per 1 mL of reaction mixture under the defined reaction conditions.2.4. Experimental design
For the optimization of xylanase production from S. thermophilum, a series of medium components and fermentation conditions were statistically investigated by RSM using the BBD method.23 Five independent variables were tested: inoculum level (X1), temperature (X2), pH (X3), carbon source (X4) and nitrogen source (X5). The range and levels of the independent variables are given in Table 1. In total, 46 experimental runs were performed in three replicates with six centre points according to the BBD matrix.| Independent variables | Variable name | Levels | ||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| X1 | Inoculum level (mL) | 1 | 2 | 3 |
| X2 | Temperature (°C) | 40 | 45 | 50 |
| X3 | pH | 5 | 6 | 7 |
| X4 | Carbon source (corn cob) (g L−1) | 20 | 30 | 40 |
| X5 | Nitrogen source (yeast extract) (g L−1) | 3 | 4 | 5 |
Minitab® Release 14 Statistical Software (USA) was used for the data analysis of the xylanase activity. A full quadratic model was adopted to predict the optimum point as shown in eqn (1):
| Y = b0 + ∑biXi + ∑biiXi2 + ∑bijXiXj | (1) |
2.5. Characterization of xylanase
2.6. Production of xylooligosaccharides
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). The precipitate was partially solubilized in 20 mL water and reprecipitated by the addition of 60 mL ethanol–acetic acid solution (10
1, v/v). The precipitate was partially solubilized in 20 mL water and reprecipitated by the addition of 60 mL ethanol–acetic acid solution (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). The precipitated hemicellulose was filtered and dried at room temperature. The recovery of hemicellulosic fraction was calculated using eqn (2):
1, v/v). The precipitated hemicellulose was filtered and dried at room temperature. The recovery of hemicellulosic fraction was calculated using eqn (2):
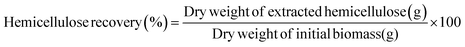 | (2) |
The extracted hemicellulose was subjected to enzymatic hydrolysis to obtain XO. After five days of S. thermophilum cultivation, the culture was filtered through Whatmann no. 1 filter paper and centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rcf. The supernatant solution was then used as crude enzyme solution. The dried substrate (biomass) was powdered using a porcelain mortar. The 10 mL reaction mixture contained 1% (w/v) biomass and 30 IU mL−1 of enzyme in 50 mM sodium phosphate buffer at pH 7.0. The reaction was carried out at 45 °C by gentle stirring. Portions of the solution were removed at 0, 1, 6 and 24 h and boiled for 10 min to stop enzyme activity. The reducing end concentrations of the samples were determined using the DNSA method with xylose as the standard.21 XO in enzymatic hydrolysates were determined by thin layer chromatography (TLC).
000 rcf. The supernatant solution was then used as crude enzyme solution. The dried substrate (biomass) was powdered using a porcelain mortar. The 10 mL reaction mixture contained 1% (w/v) biomass and 30 IU mL−1 of enzyme in 50 mM sodium phosphate buffer at pH 7.0. The reaction was carried out at 45 °C by gentle stirring. Portions of the solution were removed at 0, 1, 6 and 24 h and boiled for 10 min to stop enzyme activity. The reducing end concentrations of the samples were determined using the DNSA method with xylose as the standard.21 XO in enzymatic hydrolysates were determined by thin layer chromatography (TLC).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) solvent system. Xylose (X1), xylobiose (X2), xylotriose (X3) and xylotetrose (X4) (Megazyme, Ireland) were used as standards (2 mg mL−1 each). After development in a single step, the plate was dried and sprayed with methanol–sulfuric acid (95
1, v/v) solvent system. Xylose (X1), xylobiose (X2), xylotriose (X3) and xylotetrose (X4) (Megazyme, Ireland) were used as standards (2 mg mL−1 each). After development in a single step, the plate was dried and sprayed with methanol–sulfuric acid (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5,v/v), and the colour was developed by incubating the plate in an oven at 105 °C for several minutes. Plates were analysed using ImageJ 1.47 image processing analysis software by splitting 32 bit images into red, green and blue components. The components were tested for their signal to noise ratio (SNR) using the SNR plug-in. The red component image was chosen because it had the highest SNR. Optical density (OD) units were used to quantify the XO. XO production in percentages of the raw and the hemicellulosic portion of biomass was calculated by comparing the OD values of sample spots to the OD values of the XO standard (X1, X2, X3 and X4) spots.
5,v/v), and the colour was developed by incubating the plate in an oven at 105 °C for several minutes. Plates were analysed using ImageJ 1.47 image processing analysis software by splitting 32 bit images into red, green and blue components. The components were tested for their signal to noise ratio (SNR) using the SNR plug-in. The red component image was chosen because it had the highest SNR. Optical density (OD) units were used to quantify the XO. XO production in percentages of the raw and the hemicellulosic portion of biomass was calculated by comparing the OD values of sample spots to the OD values of the XO standard (X1, X2, X3 and X4) spots.3. Results and discussion
3.1. Optimization by response surface methodology
The culture parameters for S. thermophilum xylanase production at shake flask level were optimized by using RSM. For this purpose, a five-factorial BBD was employed. As an alternative to the commonly-used pure xylan, corn cob, which is mainly counted as an agricultural waste product, was used as the carbon source in the fermentation medium. Pure xylan is difficult to extract from lignocellulosic material, making it an expensive chemical for the fermentation process.26 Therefore, the cost of the carbon source could be marginally decreased by using corn cob. In addition, waste corn cob could be turned into a value-added raw material for fermentation.To statistically investigate the effect of important fermentation conditions (inoculum level, temperature and pH) and medium components (carbon and nitrogen sources) on response (xylanase activity), 46 experimental sets were performed. Six out of 46 sets were centre point repetitions. The experimental design and xylanase activity results with corresponding residual values are given in Table 2. Different levels of xylanase activity ranging from 1.86 to 106.02 IU mL−1 were observed. The residual plots revealed a normal distribution in the experimental data, and 41 out of 46 data points were in the residual range of −10 to +10 (data not shown).
| Run | Inoculum level (X1) | Temperature (X2) | pH (X3) | Carbon source (X4) | Nitrogen source (X5) | Experimental xylanase activity (IU mL−1) | Predicted xylanase activity (IU mL−1) | Residual |
|---|---|---|---|---|---|---|---|---|
| 1 | −1 | −1 | 0 | 0 | 0 | 44.100 | 38.130 | 5.97 |
| 2 | −1 | 1 | 0 | 0 | 0 | 85.410 | 80.575 | 4.835 |
| 3 | 1 | −1 | 0 | 0 | 0 | 46.980 | 43.052 | 3.928 |
| 4 | 1 | 1 | 0 | 0 | 0 | 43.960 | 41.167 | 2.793 |
| 5 | 0 | 0 | −1 | −1 | 0 | 49.930 | 43.446 | 6.484 |
| 6 | 0 | 0 | −1 | 1 | 0 | 3.730 | −4.253 | 7.983 |
| 7 | 0 | 0 | 1 | −1 | 0 | 54.880 | 60.422 | −5.542 |
| 8 | 0 | 0 | 1 | 1 | 0 | 62.070 | 66.114 | −4.044 |
| 9 | 0 | −1 | 0 | 0 | −1 | 31.830 | 29.445 | 2.385 |
| 10 | 0 | −1 | 0 | 0 | 1 | 46.310 | 51.693 | −5.383 |
| 11 | 0 | 1 | 0 | 0 | −1 | 25.440 | 29.350 | −3.91 |
| 12 | 0 | 1 | 0 | 0 | 1 | 80.670 | 92.348 | −11.678 |
| 13 | −1 | 0 | −1 | 0 | 0 | 9.810 | 20.959 | −11.149 |
| 14 | −1 | 0 | 1 | 0 | 0 | 72.680 | 71.990 | 0.69 |
| 15 | 1 | 0 | −1 | 0 | 0 | 7.020 | 11.076 | −4.056 |
| 16 | 1 | 0 | 1 | 0 | 0 | 55.170 | 47.388 | 7.782 |
| 17 | 0 | 0 | 0 | −1 | −1 | 49.200 | 52.737 | −3.537 |
| 18 | 0 | 0 | 0 | −1 | 1 | 106.020 | 97.600 | 8.42 |
| 19 | 0 | 0 | 0 | 1 | −1 | 31.190 | 33.974 | −2.784 |
| 20 | 0 | 0 | 0 | 1 | 1 | 83.530 | 74.356 | 9.174 |
| 21 | 0 | 0 | 0 | 0 | 0 | 57.590 | 64.532 | −6.942 |
| 22 | 0 | 0 | 0 | 0 | 0 | 68.280 | 64.532 | 3.748 |
| 23 | 0 | 0 | 0 | 0 | 0 | 63.360 | 64.532 | −1.172 |
| 24 | 0 | −1 | −1 | 0 | 0 | 19.780 | 9.694 | 10.086 |
| 25 | 0 | −1 | 1 | 0 | 0 | 24.250 | 24.976 | −0.726 |
| 26 | 0 | 1 | −1 | 0 | 0 | 2.550 | 1.584 | 0.966 |
| 27 | 0 | 1 | 1 | 0 | 0 | 63.800 | 73.646 | −9.846 |
| 28 | −1 | 0 | 0 | −1 | 0 | 79.450 | 87.461 | −8.011 |
| 29 | −1 | 0 | 0 | 1 | 0 | 52.310 | 59.158 | −6.848 |
| 30 | 1 | 0 | 0 | −1 | 0 | 61.400 | 62.919 | −1.519 |
| 31 | 1 | 0 | 0 | 1 | 0 | 48.860 | 49.215 | −0.355 |
| 32 | 0 | 0 | −1 | 0 | −1 | 1.860 | 2.695 | −0.835 |
| 33 | 0 | 0 | −1 | 0 | 1 | 19.820 | 29.927 | −9.477 |
| 34 | 0 | 0 | 1 | 0 | −1 | 40.510 | 30.346 | 10.164 |
| 35 | 0 | 0 | 1 | 0 | 1 | 90.510 | 88.989 | 1.521 |
| 36 | −1 | 0 | 0 | 0 | −1 | 51.020 | 45.990 | 5.03 |
| 37 | −1 | 0 | 0 | 0 | 1 | 102.910 | 93.428 | 9.483 |
| 38 | 1 | 0 | 0 | 0 | −1 | 27.050 | 33.563 | −6.513 |
| 39 | 1 | 0 | 0 | 0 | 1 | 69.310 | 71.370 | −2.06 |
| 40 | 0 | −1 | 0 | −1 | 0 | 36.340 | 42.762 | −6.422 |
| 41 | 0 | −1 | 0 | 1 | 0 | 35.740 | 45.578 | −9.838 |
| 42 | 0 | 1 | 0 | −1 | 0 | 96.990 | 86.862 | 10.128 |
| 43 | 0 | 1 | 0 | 1 | 0 | 48.750 | 42.038 | 6.712 |
| 44 | 0 | 0 | 0 | 0 | 0 | 70.480 | 64.532 | 5.948 |
| 45 | 0 | 0 | 0 | 0 | 0 | 59.780 | 64.532 | −4.752 |
| 46 | 0 | 0 | 0 | 0 | 0 | 67.700 | 64.532 | 3.168 |
The results of the regression analysis for the full second order polynomial model are given in Table 3. T and p (probability) values are used to predict the significance of each coefficient as well as the interactions between the variables. As a general rule, a smaller p value (generally less than 0.05) and a larger T value correspond to a more significant coefficient.18 As shown in Table 3, constant term and linear parameters have very low p values (<0.001), indicating that the effect of these terms on the response is at the 0.1% confidence level. Quadratic terms of inoculum level (inoculum*inoculum), carbon (carbon*carbon) and nitrogen (nitrogen*nitrogen) have low T values and high p values (>0.05), indicating that their effect on the response is insignificant. Only two quadratic terms, temperature*temperature and pH*pH, were found to be significant. Among the interaction terms, five coefficients were predicted to be significant with p values less than 0.05: inoculum*temperature, temperature*pH, temperature*carbon, temperature*nitrogen, pH*carbon. Terms having p values higher than 0.05 were excluded from the model equation. Using only the significant regression parameters, the following second-order polynomial model equation (eqn (3)) was obtained:
| Y [xylanase activity (IU mL−1)] = 64.532 − 8.621X1 + 10.140X2 + 21.836X3 − 10.502X4 + 21.311X5 − 12.090X22 − 24.967X32 − 11.083X1X2 + 14.195X2X3 − 11.910X2X4 + 10.188X2X5 + 13.348X3X4 | (3) |
| Term | Coef. | SE Coef. | T | p |
|---|---|---|---|---|
| a S = 8.689; R-Sq = 94.1%; R-Sq (adj) = 89.3%. | ||||
| Constant | 64.532 | 3.547 | 18.192 | <0.001 |
| Inoculum | −8.621 | 2.172 | −3.969 | <0.001 |
| Temperature | 10.140 | 2.172 | 4.668 | <0.001 |
| pH | 21.836 | 2.172 | 10.052 | <0.001 |
| Carbon | −10.502 | 2.172 | −4.835 | <0.001 |
| Nitrogen | 21.311 | 2.172 | 9.811 | <0.001 |
| Inoculum*inoculum | −1.711 | 2.941 | −0.582 | 0.566 |
| Temperature*temperature | −12.090 | 2.941 | −4.110 | <0.001 |
| pH*pH | −24.967 | 2.941 | −8.489 | <0.001 |
| Carbon*carbon | 1.868 | 2.941 | 0.635 | 0.531 |
| Nitrogen*nitrogen | −1.733 | 2.941 | −0.589 | 0.561 |
| Inoculum*temperature | −11.083 | 4.344 | −2.551 | 0.017 |
| Inoculum*pH | −3.680 | 4.344 | −0.847 | 0.405 |
| Inoculum*carbon | 3.650 | 4.344 | 0.840 | 0.409 |
| Inoculum*nitrogen | −2.408 | 4.344 | −0.554 | 0.584 |
| Temperature*pH | 14.195 | 4.344 | 3.267 | 0.003 |
| Temperature*carbon | −11.910 | 4.344 | −2.741 | 0.011 |
| Temperature*nitrogen | 10.188 | 4.344 | 2.345 | 0.027 |
| pH*carbon | 13.348 | 4.344 | 3.072 | 0.005 |
| pH*nitrogen | 8.010 | 4.344 | 1.844 | 0.077 |
| Carbon*nitrogen | −1.120 | 4.344 | −0.258 | 0.799 |
Linear coefficients with a negative sign (i.e., inoculum level and carbon source concentration) have a negative effect on the response, whereas temperature, pH and nitrogen source concentration affect the response positively. Among the five tested independent variables, pH and nitrogen source concentration had the highest effect on xylanase activity, as indicated by their high linear coefficients of 21.826 and 21.311, respectively (Table 3).
The fitness of the proposed model equation is validated by the coefficient of determination (R2; R-Sq) and adjusted coefficient of determination (adjusted R2; R-Sq adj). R2 values approaching 100% indicate the high accuracy of the proposed model. The value of R-Sq for the model equation in this study is 94.1%, and the R-Sq adj value is 89.3%. Both of these coefficients of determination are high enough to demonstrate the strong correlation between experimental and predicted responses and thus the significance of the model. Analysis of variance (ANOVA) indicated a very low value of probability (<0.001) and a very high value of F value (19.80) (Table 4). Lack of fit was found to be insignificant with a p value of 0.093. These results indicate that the model is statistically significant.
| Source | DF | Seq SS | Adj SS | Adj MS | F | p |
|---|---|---|---|---|---|---|
| Regression | 20 | 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 890.2 890.2 |
29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 890.2 890.2 |
1494.51 | 19.80 | <0.001 |
| Linear | 5 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 494.4 494.4 |
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 494.4 494.4 |
3898.88 | 51.64 | <0.001 |
| Square | 5 | 7011.1 | 7011.1 | 1402.22 | 18.57 | <0.001 |
| Interaction | 10 | 3384.7 | 3384.7 | 338.47 | 4.48 | 0.001 |
| Residual error | 25 | 1887.4 | 1887.4 | 75.50 | ||
| Lack-of-fit | 20 | 1755.8 | 1755.8 | 87.79 | 3.34 | 0.093 |
| Pure error | 5 | 131.6 | 131.6 | 26.32 | ||
| Total | 45 | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 777.7 777.7 |
Contour plots of five different variables showed that xylanase activity increases with increasing temperature (Fig. 1a and e–g), pH (Fig. 1b, e, h and i) and nitrogen source concentration (Fig. 1d, g, i and j). However, increasing inoculum level (Fig. 1a–d) and carbon source concentration (Fig. 1c, f, h and j) had a negative effect on xylanase activity. The positive effect of temperature on xylanase production from S. thermophilum can be explained by the thermophilic nature of the microorganism, which prefers high fermentation temperatures. Accordingly, elevated xylanase activities were observed at the highest tested temperature (50 °C). An increase in culture medium pH from 5.0 to 7.0 also had a positive effect on xylanase production.
S. thermophilum xylanase is an extracellular enzyme and is therefore easily affected by pH. Organisms may prefer a neutral extracellular pH (7.0) for energy metabolism. Similarly, higher xylanase activities were achieved at high nitrogen source concentrations, as expected. In microorganisms, nitrogen is used metabolically for biomass generation and protein production. Generally, high nitrogen source concentration up to the critical toxicity level is a preferable condition.
In contrast, inoculum level and carbon source concentration had a negative effect on xylanase production. An aerobic environment is one of the basic conditions for the development of thermophilic fungi including S. thermophilum.27 As inoculum level increases, the need for oxygen also increases because of the high concentration of microorganisms in the culture. At a high inoculum level, oxygen will be rapidly consumed and stress conditions will arise. Therefore, microbial growth was possibly inhibited in the culture because of oxygen stress, resulting in decreased xylanase production. Elevated levels of carbon source are generally preferred by the organisms, but the physical structure of the carbon source predominantly affects the metabolic consumption performance. The ground corn cob particles (<2 mm in size) used as the carbon source in this study possibly exerted a shear force on the fungus micelles during shaking. A high shear force could inhibit microbial growth, and consequently, the xylanase production.
From the contour plots, the optimum culture conditions in the coded unit area were determined as: X1 = −1, X2 = +1, X3 = +1, X4 = −1 and X5 = +1. The corresponding Y value (xylanase activity) was calculated by the second-order polynomial model equation (eqn (3)) as 133.91 IU mL−1.
3.2. Verification of the theoretical predictions
To check the accuracy of the model, xylanase production was tested at the predicted optimum fermentation conditions; the maximum xylanase activity was observed on the 5th day of fermentation as 134.09 IU mL−1. This result is in clear agreement with the predicted value of 133.91 IU mL−1, demonstrating the high accuracy of the proposed model. Jatinder et al. studied the optimization of culture conditions for cellulolytic and hemicellulolytic enzymes from S. thermophilum.28 In contrast to the five-factorial design of the present work, they applied a three-factorial design (inoculum level, ammonium sulfate and pH). In our study, the additional effects of temperature, carbon and nitrogen source concentrations on xylanase were also investigated. The previous study also applied solid state fermentation conditions, whereas our study employed the submerged cultivation method.Studies on xylanase biosynthesis from other fungal strains on corn cob are summarized in Table 5. The observed levels of xylanase activity range broadly from 0.89 to 1438.7 IU mL−1. Even for different strains of the same microorganism (Thermomyces lanuginosus) grown on corn cob, xylanase production levels differ considerably from 70 to 3575 IU mL−1.29 Thus, it is clear that enzyme production level depends not only on the microorganism and carbon source, but also on other fermentation process parameters such as temperature, pH, culture volume, shaking rate, oxygen level, nitrogen source, etc. In addition, the enzyme assay method also affects the level of observed activity. As shown in Table 5, enzyme assay conditions differed in each study.30–36 The comparison could be made by comparing the initial and final activity levels before and after optimization; in this study, the optimized conditions resulted in an approximately two-fold increase in xylanase activity compared to the initial conditions.
| Fungus | Corn cob concentration in culture medium (%) (w/v) | Substrate and pH of enzyme assay | Maximum xylanase activity (IU mL−1) | Reference |
|---|---|---|---|---|
| Aspergillus flavus | 3 | Birchwood xylan, pH: 5.4 | 190 | 30 |
| Aspergillus fumigatus | 1 | Birchwood xylan, pH: 5.4 | 125 | 31 |
| 1 | Oat spelt xylan, pH: 5.0 | 19.3 | 32 | |
| Aspergillus giganteus | 1 | Birchwood xylan, pH: 6.0 | 7.54 | 33 |
| Fusarium oxysporum F3 | 2 | Oat spelt xylan, pH: 7.0 | 245.26 | 34 |
| Penicillium sclerotiorum | 1 | Birchwood xylan, pH: 6.0 | 0.89 | 35 |
| Thermomyces lanuginosus | 3 | Birchwood xylan, pH: 6.5 | 1438.7 | 36 |
| Scytalidium thermophilum | 1 | Beechwood xylan, pH: 7.0 | 133.91 | This study |
3.3. Characterization of xylanase
| Substrates | Xylanase activity (IU mL−1) | Relative xylanase activity (%) |
|---|---|---|
| Commercial | ||
| Birchwood xylan | 120.52 ± 9.86 | 100.00 ± 0.00 |
| Oat spelt xylan | 115.40 ± 5.87 | 96.00 ± 2.98 |
| Beechwood xylan | 112.18 ± 3.79 | 93.45 ± 4.50 |
| Avicel | 6.26 ± 2.47 | 5.39 ± 2.49 |
| CMC | 1.33 ± 1.33 | 1.20 ± 1.20 |
| Lignocellulosic | ||
| Wheat bran | 3.25 ± 0.36 | 100.00 ± 0.00 |
| Corn cob | 0.82 ± 0.09 | 25.10 ± 0.02 |
| Sunflower stalk | 0.20 ± 0.00 | 6.30 ± 0.69 |
| Cotton stalk | 0.15 ± 0.04 | 4.69 ± 0.58 |
3.4. Hemicellulose extraction and enzymatic production of xylooligosaccharides
The flow diagram of the process described in this study is shown in Fig. 3. Starting from the raw biomass residue, two high value products, xylanase and XO, were obtained in a single process. Hemicellulose extraction and XO production from the extracted hemicelluloses was the last step of the process. By using an alkali extraction technique, the highest hemicellulose yield was obtained from corn cob (37.7 ± 1.5%), followed by wheat bran (26.6 ± 0.8%) and cotton stalk (22.2 ± 0.6%) (Table 7). This result agrees with previous studies that predict corn cob to have the highest xylan concentration among all agricultural by-products and conclude that corn cob is thus the most suitable source for XO production.37| Biomass type | Hemicellulose extraction yield from raw biomass (%) | XO production (mg XO per g hemicellulose) | XO production (mg XO per g raw biomass) |
|---|---|---|---|
| a N.D.: No data available for xylan yield of commercial beechwood preparation. | |||
| Beechwood | N.D. | 793 | N.D. |
| Sunflower stalk | 11.1 ± 0.3 | 601 | 66.7 |
| Corn cob | 37.7 ± 1.5 | 456 | 172.1 |
| Sunflower seed shell | 10.5 ± 0.3 | 482 | 50.6 |
| Wheat bran | 26.6 ± 0.8 | 159 | 42.3 |
| Cotton stalk | 22.2 ± 0.6 | 154 | 34.2 |
| Sugar beet bagasse | 8.6 ± 0.4 | 81 | 7.0 |
In our study, the XO production profiles from hemicelluloses of different origin (corn cob, cotton stalk, sunflower stalk, sugar cane bagasse, wheat bran, sunflower seed shell and commercial beechwood) using S. thermophilum xylanase were screened, and the corresponding TLC chromatograms are illustrated in Fig. 4. The production of XO from the extracted hemicelluloses was performed at 45 °C using a 30 IU mL−1 of crude xylanase solution. The XO contents of samples taken at 0, 1, 6 and 24 h were examined in comparison to the spots of standard mixtures (ST) of 2 mg mL−1 each of xylose (X1), xylobiose (X2), xylotriose (X3) and xylotetrose (X4) (Fig. 4). TLC analysis showed that the crude enzyme solution could hydrolyse the alkaline-extracted corn cob xylan into XO with degree of polymerization (DP) values ranging from 1 to 4; the main hydrolysis products were X1 and X2.
 | ||
| Fig. 4 TLC chromatograms of xylan hydrolysis. X1, xylose; X2, xylobiose; X3, xylotriose; X4, xylotetraose. | ||
Enzymatic hydrolysis of sunflower stalk produced a similar XO profile; however, X2 was an intermediate product that disappeared with X1 production. For sunflower stalk, X4 and higher sugars were observed at higher concentrations compared to corn cob. A very low yield of XO production (only X4) was obtained from cotton stalk. Sunflower seed shell hydrolysis yielded mainly X1 and X4 without X2 and X3. After 1 h reaction time, X2 and higher sugars were obtained from commercial beechwood xylan, while only X1 and X4 were observed after 6 h. Although X1 is not an XO, it is a fermentable sugar and can be utilized in the fermentation medium for the production of xylitol, a natural food sweetener.38
Based on the nature of the lignocellulosic material, a considerable part of hemicellulose polymers could be made up of xylose (xylan), arabinose (arabinan) or mannose (mannan), which can be substituted via ether or ester bonds.39 This diversity in composition and structure between hemicellulose types could explain the different XO production profiles observed for different substrates. For example, corn cob xylan and wheat bran xylan are defined as glucuronoarabinoxylan in the literature, whereas cotton stalk and sunflower stalk are identified as glucuronoxylan structures.40,41 Uckun et al. reported that except for corn cob xylan, the other substrates (cotton stalk, rice hull, sunflower stalk and wheat straw) contain lignin residues at different levels, which might explain the inhibited xylanase activity and the resulting difference in XO yield.40
Xylose-free xylobiose (X2) was obtained from sugar beet bagasse and wheat bran. XO with DP values of 2–4 are preferred in food applications.42 XO without xylose are important in the food industry from a prebiotic point of view,15 therefore, sugar beet bagasse and wheat bran are especially regarded as high-potential XO sources. Xylan in the untreated biomass, however, could not be hydrolysed by the crude enzyme solution (data not shown), possibly because of the cellulosic barrier. This result suggests that the pre-treatment of lignocellulosic biomass (alkaline extraction in this study) into soluble xylan is required for enzymatic XO production.
XO production yields at 0, 1, 6 and 24 h were calculated, and the maximum total XO production yields (sum of X1, X2, X3 and X4 yields) are given in Table 7. The highest XO production rates were observed with commercial beechwood xylan (793 mg XO per g hemicellulose), followed by sunflower stalk, corn cob and sunflower seed shell. The XO yields in terms of mg XO per g raw (untreated) biomass showed a different profile because the hemicellulose content of each biomass is different. The highest XO yield based on raw biomass was achieved from corn cob (172.1 mg XO per g raw biomass), followed by sunflower stalk (66.7 mg XO per g raw biomass). Since the hemicellulose extraction yield of commercial beechwood xylan is unknown, the XO yield based on raw biomass could not be calculated.
Samples from reaction mixtures taken at 0, 1, 6 and 24 h were also tested for their total XO concentration (mg mL−1) and reducing end concentration (mg mL−1) (Fig. 5). The maximum total available XO were obtained from beechwood (7.93 mg mL−1 at 1 h), followed by sunflower stalk (6.01 mg mL−1 at 6 h) and corn cob (4.56 mg mL−1 at 6 h). The reducing end concentrations of these samples (2.19 ± 0.03, 4.41 ± 0.03 and 3.49 ± 0.03 mg mL−1, respectively) were lower than their XO concentrations. This can be explained by the presence of oligomers rather than X1. X1 and higher oligomers have the same number of reducing ends, therefore, close levels of XO and reducing end concentrations indicate the dominance of X1. This is the case in sunflower seed shell at 24 h, which has the highest level of reducing end concentration (4.89 ± 0.02 mg mL−1) (Fig. 5).
 | ||
| Fig. 5 Maximum total XO and corresponding reducing end concentrations after enzymatic treatment of the hemicellulosic fraction of different biomass residues. | ||
4. Conclusions
This study demonstrated the co-production of xylanase enzyme and a high-value product, XO, from lignocellulosic wastes in a single process. The findings of this study could be employed for further optimization studies of scaled-up xylanase production in bioreactor systems using various agricultural wastes, thus adding value to biomass residues. According to statistical optimization results, a high nitrogen concentration was found to induce xylanase production. Therefore, as an alternative to yeast extracts, different agricultural wastes with high nitrogen contents should be further investigated to decrease fermentation costs. The present study indicated that corn cob is the most suitable source for enzymatic XO production. It gave the highest XO yield (172.1 mg XO per g raw biomass), followed by sunflower stalk (66.7 mg XO per g raw biomass). XO without xylose are known to be important in the food industry. Among the lignocellulosic biomass tested, sugar beet bagasse and wheat bran were shown to be able to act as substrates for enzymatic XO production without xylose. Further studies on enzyme purification and process optimization for XO production are underway.Acknowledgements
The financial support for this study by The Scientific and Technological Research Council of Turkey (TUBITAK) by Project-110M615 is kindly acknowledged.References
- M. S. Singhvi, S. Chaudhari and D. V. Gokhale, R. Soc. Chem. Adv., 2014, 4, 8271–8277 CAS.
- R. Singh, V. Kapoor and V. Kumar, Braz. J. Microbiol., 2012, 43, 1545–1552 CrossRef CAS PubMed.
- C. Sanchez, Biotechnol. Adv., 2007, 27, 185–194 CrossRef PubMed.
- K. Tada, J. Horiuchi, T. Kanno and M. Kobayashi, J. Biosci. Bioeng., 2004, 98, 228–230 CrossRef CAS.
- R. A. Prade, Biotechnol. Genet. Eng. Rev., 1996, 13, 101–132 CrossRef CAS.
- T. Collins, C. Gerday and G. Feller, FEMS Microbiol. Rev., 2005, 29, 3–23 CrossRef CAS PubMed.
- M. L. T. M. Polizeli, A. C. S. Rizzatti, R. Monti, H. F. Terenzi, J. A. Jorge and D. S. Amorim, Appl. Microbiol. Biotechnol., 2005, 67, 577–591 CrossRef CAS PubMed.
- P. Katapodis, V. Christakopoulou, D. Kekos and P. Christakopoulos, Biochem. Eng. J., 2007, 35, 136–141 CrossRef CAS PubMed.
- A. Kocabas, Z. B. Ogel and U. Bakir Bolukbasi, Ann. Microbiol., 2014, 64, 75–84 CrossRef CAS.
- N. Kulkarni, A. Shendye and M. Rao, FEMS Microbiol. Rev., 1999, 23, 411–456 CrossRef CAS PubMed.
- D. Haltrich, B. Nidetzky, K. D. Kulbe, W. Steiner and S. Zupancic, Bioresour. Technol., 1996, 58, 137–161 CrossRef CAS.
- B. C. Saha, J. Ind. Microbiol. Biotechnol., 2003, 30, 279–291 CrossRef CAS PubMed.
- A. A. Aachary and S. G. Prapulla, Bioresour. Technol., 2009, 100, 991–995 CrossRef CAS PubMed.
- O. Akpinar, K. Erdogan and S. Bostanci, Food Bioprod. Process., 2009, 87, 145–151 CrossRef CAS PubMed.
- D. Chapla, S. Dholakiye, D. Madawar and A. Shah, Food Bioprod. Process., 2013, 91, 682–692 CrossRef CAS PubMed.
- N. Jayapal, A. K. Samanta, A. P. Kolte, S. Senani, M. Sridhar, K. P. Suresh and K. T. Sampath, Ind. Crops Prod., 2013, 42, 14–24 CrossRef CAS PubMed.
- Y. H. Wang, J. T. Feng, O. Zhang and X. Zhang, J. Appl. Microbiol., 2008, 104, 735–744 CrossRef CAS PubMed.
- R. H. Myers and D. C. Montgomery, Response Surface Methodology: Process and Product Optimization Using Designed Experiments, John Wiley and Sons, New York, 2002 Search PubMed.
- D. G. Cooney and R. Emerson, Thermophilic fungi, Freeman Publishers, San Francisco, 1964 Search PubMed.
- Z. B. Ogel, K. Yarangumeli, H. Dundar and I. Ifrij, Enzyme Microb. Technol., 2001, 28, 689–695 CrossRef CAS.
- M. J. Bailey, P. Biely and K. Pountanen, Biochim. Biophys. Acta, 1992, 1117, 252–270 Search PubMed.
- G. L. Miller, Anal. Chem., 1959, 31, 426–428 CrossRef CAS.
- G. Box and D. Behnken, Technometrics, 1960, 2, 455–475 CrossRef.
- C. Zilliox and P. Debeire, Enzyme Microb. Technol., 1998, 22, 58–63 CrossRef CAS.
- E. Bahcegul, B. Akinalan, H. E. Toraman, D. Erdemir, N. Ozkan and U. Bakir, Bioresour. Technol., 2013, 149, 582–585 CrossRef CAS PubMed.
- D. A. Bocchini, H. F. Alves-Prado, L. C. Baida, I. C. Roberto, E. Gomes and R. Da Silva, Process Biochem., 2002, 38, 727–731 CrossRef CAS.
- R. Maheshwari, G. Bharadwaj and M. K. Bhat, Microbiol. Mol. Biol. Rev., 2000, 64, 461–488 CrossRef CAS.
- K. Jatinder, B. S. Chadha and H. S. Saini, World J. Microbiol. Biotechnol., 2006, 22, 169–176 CrossRef CAS.
- S. Singh, A. M. Madlala and B. A. Prior, FEMS Microbiol. Rev., 2003, 27, 3–16 CrossRef CAS.
- C. G. M. De Souza, N. G. Girardo, M. A. F. Costa and R. M. Peralta, J. Basic Microbiol., 1999, 39, 155–160 CrossRef CAS.
- V. Lenartovicz, C. G. M. De Souza, F. G. Moreira and R. M. Peralta, J. Basic Microbiol., 2002, 42, 388–395 CrossRef CAS.
- T. Anthony, K. C. Raj, A. Rajendran and P. Gunasekaran, Enzyme Microb. Technol., 2003, 32, 647–654 CrossRef CAS.
- G. D. Coelho and E. C. Carmona, J. Basic Microbiol., 2003, 43, 269–277 CrossRef CAS PubMed.
- P. Christakopoulos, W. Nerinckx, D. Kekos, B. Macris and M. Claeyssens, J. Biotechnol., 1996, 51, 181–189 CrossRef CAS.
- A. Knob and E. C. Carmona, World Appl. Sci. J., 2008, 4, 277–283 Search PubMed.
- J. Gomes, H. Purkarthofer, M. Hayn, J. Kapplmiller, M. Sinner and W. Steiner, Appl. Microbiol. Biotechnol., 1993, 39, 700–707 CrossRef CAS.
- D. O. Otieno and B. K. Ahring, Carbohydr. Res., 2012, 360, 84–92 CrossRef CAS PubMed.
- P. Jeevan, R. Nelson and A. E. Rena, J. Microbiol. Biotechnol. Res., 2011, 1, 114–123 CAS.
- M. J. Vazquez, J. L. Alonso, H. Dominguez and J. C. Parajo, Trends Food Sci. Technol., 2000, 11, 387–393 CrossRef CAS.
- E. Uckun Kiran, O. Akpinar and U. Bakir, Food Bioprod. Process., 2013, 91, 565–574 CrossRef CAS PubMed.
- M. E. F. Schooneveld-Bergmans, Y. M. van Dijk, G. Beldman and A. G. J. Voragen, J. Cereal Sci., 1999, 29, 49–61 CrossRef CAS.
- Q. Yan, S. Hao, Z. Jiang, Q. Zhai and W. Chen, J. Mol. Catal. B: Enzym., 2009, 58, 72–77 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |



