Determination of adsorption mechanism of polycarboxylate-ether based superplasticizers using crystallization, thermal and mass spectrometry methods
Guoxing Suna,
Ling Wang*b,
Lu-Tao Wengcd,
Jinrui Zhanga,
Zongjin Li*a and
Guangming Chene
aDepartment of Civil and Environmental Engineering, The Hong Kong University of Science and Technology, Clear Water Bay, Hong Kong, P.R. China. E-mail: zongjin@ust.hk; Fax: +852-2358-1534; Tel: +852-2358-8751
bChina Building Materials Academy, Beijing 100024, P.R. China. E-mail: wangling@cbmamail.com.cn
cMaterials Characterization and Preparation Facility, The Hong Kong University of Science and Technology, Clear Water Bay, Hong Kong, P.R. China
dDepartment of Chemical and Biomolecular Engineering, The Hong Kong University of Science and Technology, Clear Water Bay, Hong Kong, P.R. China
eBeijing National Laboratory for Molecular Sciences (BNLMS), Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, P.R. China
First published on 20th May 2014
Abstract
In this study, a series of suspensions were fabricated by dispersing calcium carbonate (CaCO3), cement and silica fume into a polycarboxylate-ether plasticizer (PCE)/water solution. The PCE used was a comb-like copolymer containing a sodium polymethacrylate (PMA) backbone partially esterified with polyethyleneglycol (PEG) side chains. Sedimentation and optical microscopy tests indicated that both CaCO3 and cement could form homogeneous suspensions. The crystallization behavior of the PEG side chains revealed that PEG had stronger interactions with CaCO3 than with cement and silica fume particles, which was further confirmed by differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA). A detailed time-of-flight secondary ion mass spectrometry (ToF-SIMS) examination suggested that PEG was mainly located on the surfaces of the CaCO3, and the PMA backbones were mainly located on the surfaces of the cement and silica fume, respectively. The different interactions between copolymer and inorganic particles were associated with their interfacial tensions, and had a remarkable influence on the paste fluidity.
Introduction
In the past three decades, polycarboxylate-ether based superplasticizers (PCEs) have attracted considerable attention due to their excellent water-reducing capability, which makes them an ideal candidate of water reducer in the manufacturing of high-performance concrete.1,2 A typical PCE structure is a comb-like copolymer with a sodium polymethacrylate (PMA) backbone partially esterified with polyethyleneglycol (PEG) chains.3 It has been proven that the increased fluidity of cement paste provided by PCE is due to the adsorption of PCE on cement particles,4 which imposes a strong static electrical field to enforce the separation of cement particles.5,6 Furthermore, the dispersion of cement particles is amplified by the steric repulsion and hydrophilic imparting effect of the PEG side chains.4,7Calcium carbonate (CaCO3) and silica fume are two important cementitious minerals in concrete.2,8–10 The addition of these two minerals significantly influences the fluidity of cement pastes. The interaction between the polymer and inorganic surfaces is important for paste fluidity. However, to the best of our knowledge, the interaction between PCE and inorganic binders, such as CaCO3 and silica fume, has not been reported yet. Normally, in polymer/inorganic composite systems, the interfacial force is weak11,12 unless chemical bonds form at the interface.13–15 For the adsorption of PCE on the inorganic surface, the exact adsorption mechanism is unclear. Since PCE is a copolymer with a PMA backbone and PEG side chain, the different charge and surface tension of these two components might contribute to the adsorption mechanism.
Although various techniques, such as rheology, atomic force microscopy (AFM), and ζ-potential, have been used to characterize the effect of PCE on cement suspensions,16,17 the crystallization behavior of PCE has seldom been emphasized in the investigation of adsorption mechanism. Note that in the PCE molecular structure, the PMA backbone is non-crystallizable due to the steric effect of its side groups but PEG is a typical semi-crystalline polymer.18 During either melt- or solvent-induced crystallization processes, PEG can develop a spherulitic morphology, which can be clearly observed with polarized optical transmission microscopy (POM).19 Under heterogeneous nucleation conditions, the number and size of the spherulites are significantly influenced by the contacted substrate. Therefore, we can identify the interaction between PCE and inorganic surfaces from the crystallization morphology of the side chains. Moreover, the thermal properties of semi-crystallized PCE, such as its endothermic enthalpy and thermal stability, during the heating process can also be used to reflect its interaction with inorganic surfaces because the strong interaction between polymer and inorganic surfaces can restrict the mobility of polymer chains and thus influence the crystallinity and degradation temperature.
When PCE is adsorbed on different inorganic surfaces and forms a monolayer, the surface concentration of PMA backbone and PEG side chain is controlled by different adsorption groups. Thus, a detailed surface analysis can be employed to determine which group of PCE is adsorbed on the relevant inorganic surface. In addition to electrostatic attraction, interfacial effect plays an important role in the adsorption of different groups. Normally, a substrate tends to adsorb groups that have smaller interfacial tension.
In this study, we investigated the adsorption mechanism of PCE on the surfaces of CaCO3, cement, and silica fume. First, the dispersibility and stabilization of inorganic particles in PCE solutions were characterized by sedimentation and optical microscopy (OM) tests. Then, the crystallization behavior of PCE and detailed thermal and surface properties of PCE/inorganic composites were studied using polarized optical microscopy (POM), differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), and time-of-flight secondary ion mass spectrometry (ToF-SIMS). Finally, the surface and interfacial tensions of polymer and inorganic particles were examined by contact angle measurement.
Materials and methods
Materials
PCE/water solution with a concentration of 21.5 wt% was produced by Grace Concrete Admixture Products. Calcium carbonate lime stone powder (CaCO3) was obtained from Jiangxi Guangyuan Chemical Co., Ltd. The particle sizes were 325, 600, 800 and 1250 meshes, and named as C325, C600, C800, and C1250, respectively. The average particle sizes of these four CaCO3 samples were measured to be around 18, 12, 8, and 5 μm, respectively, using an OMEC LC908 laser particle size analyzer. Cement that satisfies the requirements of BS EN197-1:2000 (a European standard that was adopted as a British Standard) for CEM I Portland cement of strength class 52.5 N (roughly equivalent to the requirements of ASTM C150 for Type I Portland cement) is used in this study. Silica fume particles were obtained from Elkem ASA.Methods
Inorganic particles (CaCO3, cement, and silica fume) were dispersed in PCE/water 21.5 wt% solution. The weight ratio of the inorganic particles in the solution was 4%. The mixtures were magnetically stirred for 20 min and then ultrasonicated for 10 min. Some of the suspensions were first dropped onto a glass slide for OM characterization, and then dried in a vacuum oven at room temperature for 3 days for POM characterization.The remaining suspensions were dried in a dish in a vacuum oven at room temperature for 3 days to evaporate the solvent, then the dried paste was ground into powder and used for DSC and TGA characterizations with a heating rate of 10 °C per minute in a nitrogen atmosphere. To prepare the specimens for ToF-SIMS examination, CaCO3, cement, and silica fume were pressed into flat plates at 100 MPa. Then, the PCE/water 21.5 wt% solution was spin-coated onto the plates at a spin rate of 3000 rpm. The contact angle measurements were performed at room temperature with a G10 contact angle analyzer (Krüss GmbH Co., Germany) equipped with a video capture module.
Results and discussion
Dispersibility and stabilization of inorganic particles in PCE solutions
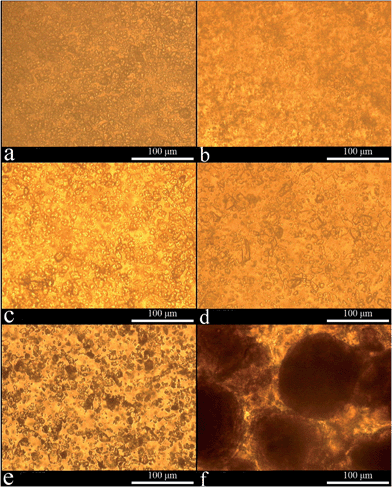
The photographs of the vials of the suspensions are shown in Fig. 1. Both CaCO3 and cement achieved uniform dispersion in PCE solution, though some solute still precipitated automatically at the bottom, as shown in Fig. 1a to e. Uniform and stable white dispersions were clearly visible in CaCO3 dispersed in PCE solution. Further careful observations revealed that the color darkened with the decrease of CaCO3 meshes (cf. Fig. 1a to d). Moreover, in cement suspension, the color turned darker from the top to the bottom (cf. Fig. 1e). Precipitations at the bottom together with the upper clear solution phase can be observed in Fig. 1f, indicating that PCE solution is not a good dispersing medium for silica fume. The silica fume precipitation layer was at the highest height, which shows that the interaction between PCE and silica fume was the poorest in this investigation.Fig. 2 shows the optical transmission micrographs of the dried film samples of the abovementioned CaCO3, cement, and silica fume dispersions. Fig. 2a to e are for CaCO3 and cement where no obvious large agglomerates but clear individual particles can be observed. The particle sizes of CaCO3 with different meshes observed from the optical images are consistent with the previously mentioned laser particle size analyzer result. This demonstrates that the good dispersibility of CaCO3 in PCE solution is not much influenced by particle sizes. Normally, the particle sizes of CaCO3 influence only the amount of adsorbed PCE. Thus, in the following study, we focus only on the CaCO3 of 1250 mesh. A high degree of silica fume aggregation is observed, and large agglomerates with an average size of 100 μm dominate, as shown in Fig. 2f. Note that in the drying process of the suspensions, it is possible that the debundled inorganic particles reaggregated into agglomerates due to large surface tension. Large agglomerates were observed in Fig. 2a–e. This provides further evidence that PCE solution significantly improves the dispersion and the stability of CaCO3 and cement, leading to the absence of large agglomerates. This is consistent with the abovementioned sedimentation experimental results, and similar deductions can be found in our previous work.20
Crystallization morphology of PCE side chains
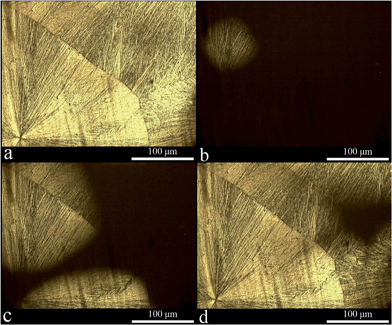
Before demonstrating how PCE molecules interact with the inorganic particles, the molecular structure of PCE is described below in detail. The PCE structure used is a comb-like copolymer made of a sodium polymethacrylate (PMA) backbone partially esterified with polyethyleneglycol (PEG). The chemical structure of the PCE molecule is shown below:16It is well-known that PMA is non-crystallizable due to the steric effect of its side groups, while PEG is a typical semi-crystalline polymer.18 During either melt or a solvent-induced crystallization process, PEG develops a spherulitic morphology, which can be clearly observed using a polarized optical transmission microscopy (POM).19 As shown in Fig. 3a, when PCE solution is dried on a glass slide at room temperature and forms a polymer film around 50 μm thick, clear spherulites with the diameters of around 400 μm can be observed under POM. Normally, spherulite development depends on how it is nucleated. A common progression is that a spherulite begins with a fibre and evolves through sheaf-like embryos before attaining a spherical envelope. Adjacent growing spherulites impinge on each other, forming polygonal shapes, exactly like the single-crystalline grains of a metal.21,22 When the solvent-induced spherulites in the film were heated to 80 °C, which 20 °C higher than the melting temperature of pure PEG,19 the spherulites completely melted and the window under POM turned dark. After that, when the same film was cooled to 30 °C, amorphous PEG chains started to form crystals and the spherulites appeared again (cf. Fig. 3b to d). However, the melt-crystallized spherulites did not grow from the center similar to the common case mentioned above. Instead, bright crystallized parts appeared randomly in the spherulite region of Fig. 3a (cf. Fig. 3b to d). Tsitsilianis et al. had proved that in comb-like copolymers, the crystallizable part could be phase-separated from the non-crystallizable part during the crystallization process.23 When PCE dried from solution, PEG crystallized and was phase-separated from PMA. At this state, PMA was in a solution state and did not disturb the formation of PEG spherulites (cf. Fig. 3a). However, when the PEG spherulites were melted and cooled, PMA was in a solid state because its glass transition temperature was 130 °C.18 Thus, PEG crystallized in a restricted environment due to the indurated PMA and only formed tiny crystals in the previous spherulite region. This demonstrated how PEG crystals randomly formed in the spherulite region of Fig. 3a (cf. Fig. 3b to d).
From the abovementioned detailed discussion of the crystallization behavior of PCE, the interaction between PCE and CaCO3, cement, or silica fume can be examined. It is obvious that it would be more desirable if the interaction could be studied in solution. Unfortunately, very few analytical techniques are available to study the interaction in liquid samples. Therefore, we carried out this analysis on dried samples and expected that the interaction can be indirectly obtained from these samples. The heterogeneous nucleation during the formation of PEG spherulites is employed in the following discussion. In Fig. 3, a PCE solution with no impurities was dropped onto a clean glass slide. During the solvent-induced crystallization process, the heterogeneous nucleation effect was weak and the PEG formed spherulites had a diameter of 400 μm. When the suspensions of PCE and CaCO3, cement, or silica fume were dried and spherulites were formed because of the heterogeneous nucleation effect of these inorganic particles, the number of spherulites increased and there size decreased as compared to those in Fig. 3 (cf. Fig. 4a, c and e). Among the three PCE/inorganic particle systems, CaCO3 had the strongest heterogeneous nucleation effect due to the large number and small size of PEG spherulites from PCE/CaCO3 suspension (cf. Fig. 4a). In contrast, fewer large spherulites were generated during the drying process of the PCE/cement and PCE/silica fume suspensions (cf. Fig. 4c and e). The interaction of PEG side chains with cement or silica fume was weaker than that of CaCO3. The POM images in Fig. 4b, d and f were obtained by heating the samples in Fig. 4a, c and e to 80 °C, then cooling to 30 °C and keeping for 60 min. As can be seen from the figures, during the melt-crystallization process, spherulites became darker and indistinct, indicating that the crystallinity of PEG decreased. It was proven that the mobility of polymer chains was restricted within 30 nm to the solid substrate.24 During the melt-crystallization process, both the indurated PMA and inorganic particles restricted the PEG chain mobility and decreased its crystallinity. The spherulites formed in the PCE/CaCO3 system were the darkest (cf. Fig. 4b), indicating that the crystallinity of PEG was the lowest, thus the contact of PEG to CaCO3 was stronger than that of cement and silica fume.
Thermal analysis of PCE/inorganic composites
To confirm the abovementioned results inferred from the crystallization morphology of PEG, a DSC test was employed to study the heating and cooling process of PCE and PCE/inorganic composites dried from the solution. As shown in Fig. 5a, during the heating process, an endothermic peak around 50 °C can be observed in both PCE and the composites. This peak represents the melting of PEG crystals that were formed during the drying process. In Table 1, we can see that the endothermic enthalpy of PCE/CaCO3 is 89.1 J g−1, which is much lower than the others. This demonstrates that the crystallinity of PEG is lower than the others when it interacts with CaCO3 during the solvent-induced crystallization process. Fig. 5b shows the cooling process of PCE and the composites. PCE generates an exothermic peak at 19.7 °C, which is about 10 °C lower than that of the other three composites. The reason is that the heterogeneous nucleation of pure PCE is weak, and thus it is difficult for PCE to crystallize at high temperature. However, with the interaction of CaCO3, melt-crystallization can occur even at a temperature of 29.8 °C, which is also higher than the exothermic peak temperatures of the composites containing cement and silica fume. This provides further evidence that CaCO3 has the strongest interaction with PEG side chains when it is dispersed in a PCE solution. The strong interaction between PEG and CaCO3 also caused the lowest crystallinity of PEG during the melt-crystallization process, as demonstrated by the lowest exothermic enthalpy of 68.7 J g−1 (cf. Table 1).| Specimen | Endothermic peak temperatures and associated enthalpies | Exothermic peak temperatures and associated enthalpies | ||
|---|---|---|---|---|
| Tp (°C) | ΔH (J g−1) | Tp (°C) | ΔH (J g−1) | |
| PCE | 51.5 | 108.0 | 19.7 | 83.7 |
| PCE/CaCO3 | 49.6 | 89.1 | 29.8 | 68.7 |
| PCE/cement | 51.1 | 105.9 | 27.8 | 82.2 |
| PCE/silica fume | 50.1 | 101.3 | 25.5 | 77.8 |
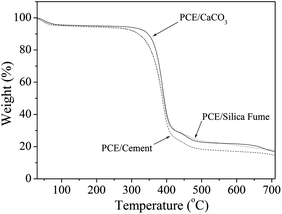
The difference in the interaction between PCE and different inorganic particles can also be seen from TGA tests. As shown in Fig. 6, the onset decomposition temperature (Tonset) and the temperature at the maximum degradation rate (Tmax, determined from the derivative of TGA curve) of PCE/CaCO3 is 364 and 390 °C, respectively. In contrast, both PCE/cement and PCE/silica fume under the same processing condition show much lower Tonset (355 °C) and Tmax (376 °C). The increase in the Tonset and Tmax are 9 and 14 °C, respectively. The strong interaction between PCE and CaCO3 may prevent polymer chains from debundling and degradation, and significantly improve thermal stability.
Interfacial effect between PCE and inorganic surfaces
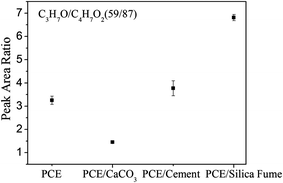
The strong interaction between CaCO3 and PEG side chains suggests that most PEG side chains adsorbed onto the CaCO3 surface instead of PMA backbone when CaCO3 was mixed with PCE solution. To investigate the distributions of PEG side chains and PMA backbone on the surfaces of inorganic particles, ToF-SIMS was used to determine the surface chemical composition of the PCE-coated inorganic plates. Positive ion C3H7O+ was chosen to represent the PEG side chains, while C4H7O2+ was chosen to represent the PMA backbone. The peak area ratio of C3H7O+ to C4H7O2+ was used to represent the concentration ratio of PEG to PMA. The ToF-SIMS results of the specimens of PCE/water 21.5 wt% solution spin-coated on the surfaces of CaCO3, cement, and silica fume plates showed that the PEG-to-PMA ratio on the surface of the CaCO3 plate was much lower than that on the surface of the pure PCE, cement, and silica fume plates (cf., Fig. 7). Therefore, from the surface chemical composition of these PCE-coated inorganic plates, we can conclude that most PEG side chains adsorbed onto the CaCO3 surface, while the PMA backbone mainly adsorbed onto the surface of cement and silica fume. The higher PEG-to-PMA ratio on cement surface than that of the neat PCE surface partially supports the argument that in the cement paste, PCE adsorbs onto the positively charged surface sites of the cement particles with their anionic trunk polymer chain. The part of the cement particles carrying a cationic charge is neutralized by the PCE.7To elucidate the mechanism of the adsorption of PCE on the surfaces of CaCO3, cement, and silica fume, the interfacial effect between PCE or PMA and inorganic particles was investigated in detail. In order to obtain the interfacial tensions between polymer and inorganic substrates, the relevant surface tensions were measured using a contact angle method. For PEG and PMA, the contact angle θ can be easily obtained by dropping the test liquids onto the as-formed polymer films. However, since CaCO3, cement and silica fume are all powder samples, the relevant contact angle θ needs to be calculated using the Washburn eqn (1):25
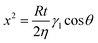 | (1) |
| Test liquid | Viscosity (η) | Surface tension (mJ m−2) | ||
|---|---|---|---|---|
| Dispersive (γdl) | Polar (γpl) | Total (γl) | ||
| n-Hexane | 0.307 | 18.43 | 0 | 18.43 |
| Water | 0.8937 | 21.8 | 51.0 | 72.8 |
| Ethyl glycol | 20.93 | 30.9 | 16.8 | 47.7 |
The surface tensions of polymer and inorganic powder are calculated using the geometric-mean eqn (2):27
γl(1 + cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ) = 2[(γdlγds)1/2 + (γplγps)1/2], θ) = 2[(γdlγds)1/2 + (γplγps)1/2],
| (2) |
| Material | Surface tension (mJ m−2) | ||
|---|---|---|---|
| Dispersive (γds) | Polar (γps) | Total (γs) | |
| PEG | 8.2 ± 0.5 | 32.1 ± 1.2 | 40.3 ± 0.9 |
| PMA | 14.2 ± 0.3 | 32.9 ± 0.5 | 47.1 ± 0.7 |
| CaCO3 | 2.9 ± 0.3 | 56.8 ± 1.1 | 59.7 ± 1.0 |
| Cement | 502.3 ± 3.2 | 25.9 ± 1.5 | 528.2 ± 2.6 |
| Silica fume | 17.7 ± 0.6 | 26.2 ± 1.8 | 43.9 ± 1.3 |
The interfacial tension between the polymer and inorganic substrate, γ1,2, is calculated using the geometric-mean eqn (3):27
 | (3) |
| Interface tension (mJ m−2) | Polymers | |
|---|---|---|
| PEG | PMA | |
| CaCO3 | 4.8 ± 0.3 | 7.5 ± 0.8 |
| Cement | 382.5 ± 3.7 | 348.0 ± 5.2 |
| Silica fume | 2.10 ± 0.1 | 0.57 ± 0.2 |
It is clear from the abovementioned crystallization and thermal results that the interaction of PCE with CaCO3 is stronger than those with cement and silica fume. This is in agreement with the dispersibility measurements of these inorganic particles in PCE solution. Furthermore, based on the findings obtained from the ToF-SIMS and interfacial energy measurements, the detailed adsorption mechanism of PCE on the surfaces of inorganic particles can be deduced. The backbone and side chain of PCE adsorb on the surfaces of cement and CaCO3, respectively due to the different interfacial tension. This finding would be very useful for the selection criterion of inorganic fillers and to identify their compatibility with superplasticizers. In addition, if the adsorption behavior of the superplasticizer on the surfaces of selected inorganic fillers is determined, their influence on fluidity can be predicted. For example, based on the adsorption behavior of PCE on the surfaces of cement and CaCO3, the addition of CaCO3 to cement will not reduce the fluidity of cement paste, as demonstrated by the fluidity test described in the following section.
Fluidity test
The performance of different adsorptions of PCE on the surfaces of CaCO3 and cement can be seen from the fluidity of the cement/CaCO3 paste. Fluidity was determined by the spread diameter of cement paste with the presence and absence of CaCO3 after the slump cone was lifted. As shown in Fig. 8, the spread diameter of cement paste apparently decreases with time, indicating that the fluidity decreases during the hydration process. With the addition of 20 wt% and 30 wt% CaCO3, represented by cement/CaCO3 ratio (80/20) and (70/30), respectively, the fluidity decrease with time is obviously reduced. The dosage of PCE/water solution (21.5 wt%) in cement, cement/CaCO3 (80/20) and (70/30) was 0.40 wt%, which is decided by controlling the initial spread diameter to be 260 ± 5 mm. This demonstrated that CaCO3 could improve the fluidity of cement paste. Since CaCO3 mainly adsorbs the PEG side chains, while cement mainly adsorbs the PMA backbone, the addition of CaCO3 did not influence the interaction between cement and PCE. Further, the steric repulsion of PEG side chains was amplified due to the adsorption on CaCO3 particles, resulting in an increase of dispersion of cement particles. Therefore, the fluidity of cement/CaCO3 had been improved dramatically during the cement hydration process.Conclusions
The adsorption mechanism of polycarboxylate-ether based superplasticizers on the surface of CaCO3, cement, and silica fume were investigated using several methods in this study. The strong interaction between CaCO3 and the PEG side chains of the superplasticizer was first demonstrated by the crystallization behavior of PEG. In this work, ToF-SIMS was used to prove that CaCO3 mainly adsorbed PEG side chains, while cement and silica fume mainly adsorbed PMA backbone. Thus, CaCO3 could improve the fluidity of cement paste. The adsorption mechanism was explained by an interfacial effect based on the measured interfacial tensions between the superplasticizer and inorganic surfaces. This work opens new avenues for the future analysis of mechanism of superplasticizers and to the selection criterion of inorganic fillers.Acknowledgements
The financial support from Hong Kong Research Grants Council under the grant of 615412 is gratefully acknowledged.References
- B. H. Guan, Q. Q. Ye, J. L. Zhang, W. B. Lou and Z. B. Wu, Cem. Concr. Res., 2010, 40, 253–259 CrossRef CAS PubMed.
- J. Plank, C. Schroefl, M. Gruber, M. Lesti and R. Sieber, J. Adv. Concr. Technol., 2009, 7, 5–12 CrossRef CAS.
- S. Pourchet, S. Liautaud, D. Rinaldi and I. Pochard, Cem. Concr. Res., 2012, 42, 431–439 CrossRef CAS PubMed.
- H. Uchikawa, S. Hanehara and D. Sawaki, Cem. Concr. Res., 1997, 27, 37–50 CrossRef CAS.
- Z. Li, Advanced concrete technology, Wiley, Hoboken, N.J., 2011 Search PubMed.
- D. Hou, H. Ma, Y. Zhu and Z. Li, Acta Mater., 2014, 67, 81–94 CrossRef CAS PubMed.
- J. Ruhe, M. Ballauff, M. Biesalski, P. Dziezok, F. Grohn, D. Johannsmann, N. Houbenov, N. Hugenberg, R. Konradi, S. Minko, M. Motornov, R. R. Netz, M. Schmidt, C. Seidel, M. Stamm, T. Stephan, D. Usov and H. N. Zhang, Polyelectrolite Brushes, in Polyelectrolytes with Defined Molecular Architecture I, Advances in Polymer Science, ed. M. Schmidt, Springer, 2004, pp. 79–150 Search PubMed.
- P. De Silva, L. Bucea and V. Sirivivatnanon, Cem. Concr. Res., 2009, 39, 460–465 CrossRef CAS PubMed.
- T. Matschei, B. Lothenbach and F. P. Glasser, Cem. Concr. Res., 2007, 37, 551–558 CrossRef CAS PubMed.
- C. Schrofl, M. Gruber and J. Plank, Cem. Concr. Res., 2012, 42, 1401–1408 CrossRef PubMed.
- G. X. Sun, G. M. Chen, J. Liu, J. P. Yang, J. Y. Xie, Z. P. Liu, R. Li and X. Li, Polymer, 2009, 50, 5787–5793 CrossRef CAS PubMed.
- G. X. Sun, G. M. Chen, Z. P. Liu and M. Chen, Carbon, 2010, 48, 1434–1440 CrossRef CAS PubMed.
- H. Y. Ma, Y. Tian and Z. J. Li, J. Mater. Civ. Eng., 2011, 23, 1412–1421 CrossRef CAS.
- H. Ma and Z. Li, Construct. Build. Mater., 2013, 47, 579–587 CrossRef PubMed.
- A. Stewart, B. Schlosser and E. P. Douglas, ACS Appl. Mater. Interfaces, 2013, 5, 1218–1225 CAS.
- L. Ferrari, J. Kaufmann, F. Winnefeld and J. Plank, Cem. Concr. Res., 2011, 41, 1058–1066 CrossRef CAS PubMed.
- K. Yamada, Cem. Concr. Res., 2011, 41, 793–798 CrossRef CAS PubMed.
- J. E. Mark and Books24x7 Inc., Oxford University Press, Oxford; Hong Kong, 2nd edn, 2009, vol. vii, p. 1240, p. 1, online resource.
- C. Nakafuku and M. Sakoda, Polym. J., 1993, 25, 909–917 CrossRef CAS.
- G. X. Sun, Z. P. Liu and G. M. Chen, NANO, 2010, 5, 103–109 CrossRef CAS.
- D. C. Bassett, in Cambridge solid state science series, Cambridge University Press, Cambridge, 1981 Search PubMed.
- G. X. Sun, L.-T. Weng, J. M. Schultz and C. M. Chan, Polymer, 2014, 55, 1829–1836 CrossRef CAS PubMed.
- C. Tsitsilianis, D. Papanagopoulos and P. Lutz, Polymer, 1995, 36, 3745–3752 CrossRef CAS.
- Y. Wang, C. M. Chan, K. M. Ng and L. Li, Macromolecules, 2008, 41, 2548–2553 CrossRef CAS.
- P. M. Costanzo, W. Wu, R. F. Giese and C. J. Vanoss, Langmuir, 1995, 11, 1827–1830 CrossRef CAS.
- I. M. Smallwood, Handbook of organic solvent properties, Arnold, London, 1996, vol. xxi, p. 306 Search PubMed.
- D. K. Owens and R. C. Wendt, J. Appl. Polym. Sci., 1969, 13, 1741–1747 CrossRef CAS.
- G. X. Sun and C. M. Chan, Colloid Polym. Sci., 2013, 291, 1495–1501 CAS.
| This journal is © The Royal Society of Chemistry 2014 |