Thermal-healable and shape memory metallosupramolecular poly(n-butyl acrylate-co-methyl methacrylate) materials†
Abstract
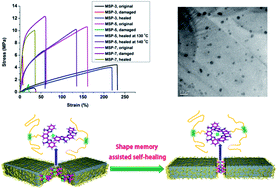
A big challenge in developing stimuli-responsive materials is to integrate multiple functionalities such as shape memory property, healable ability, recyclability into a single-component material. With this purpose, we designed a novel poly(n-butyl acrylate-co-methyl methacrylate) bearing a side group 2,6-bis(1′-methylbenzimidazolyl)pyridine ligand, which is dynamically crosslinked by the metal salt zinc trifluoromethanesulfonate to obtain the metallosupramolecular polymer. The shape recovery and healing is achieved upon application of a thermal or light stimulus due to the specific metal–ligand interactions which not only serve as an “inert” crosslink network at low temperature to produce the shape recovery, but also dissociate at high temperature for healing. The healing rate is quick and the healing efficiently is close to ∼90%.

 Please wait while we load your content...
Please wait while we load your content...