Copper-catalyzed cascade reactions of N-(2-bromoallyl)amines with KHCO3 as the C1 source: an efficient process for the synthesis of oxazolidin-2-ones†
Hongwei Jin,
Yukun Yang,
Jianhong Jia,
Binjie Yue,
Bo Lang and
Jianquan Weng*
College of Chemical Engineering, Zhejiang University of Technology, Hangzhou 310014, P. R. China. E-mail: jqweng@zjut.edu.cn; Fax: +86-0571-88320220
First published on 28th April 2014
Abstract
A novel synthesis of oxazolidin-2-ones by carbamic acid formation and a subsequent copper-catalyzed intramolecular vinylation from N-(2-bromoallyl)amines and KHCO3 was developed. KHCO3 was used as a C1 source and base in this efficient and convenient cascade process.
As an abundant, cheap and nontoxic C1 source for the production of organic chemicals, carbon dioxide is attractive to both industrial and academic scientists.1 Extensive research has been conducted on the transformation of carbon dioxide into useful bulk products.2 Nevertheless, the critical conditions regarding the transportation and storage of carbon dioxide as well as the safety factors associated with high-pressured batch reaction processes affect its utilization in scientific research and industrial production to some extent. Meanwhile, as a safe, cheap and commercially available carbon dioxide equivalent, inorganic carbonate is rarely used in organic synthesis as a C1 source, and its application is undoubtedly desirable.3
The classical copper-mediated Ullmann coupling reaction has been developed over one hundred years,4 and great achievements have been made in the copper-mediated C–N, C–O and C–C bond formation reactions in the past two decades.5 Although various ethers and oxygenated heterocycles can be constructed using a copper-mediated C–O formation reaction,6 to our knowledge results have rarely been reported on the copper-mediated vinylation of carboxylic acid.7 During the course of our continued research on the copper-catalyzed synthesis of heterocycles,8 we unexpectedly discovered a novel cascade reaction of N-(2-bromoallyl)amine with KHCO3, in which KHCO3 acted as a C1 source, leading to the formation of oxazolidin-2-ones in moderate to good yields. The resulting heterocycles are important ubiquitous substructural units which exist in many natural products and biologically active compounds,9 and can be obtained from CO2 fixing reactions.10
Using 0.2 equivalent of CuI as the catalyst and 0.4 equivalent of L-proline as the ligand, N-(2-bromoallyl)benzylamine (1a) was treated with 2 equivalents of K2CO3 in DMSO at 120 °C for 2 hours. After analyzing the 13C-NMR and IR spectra of the final product, we surprisingly found that oxazolidin-2-one A (45% yield) was obtained instead of the expected aziridine B through a direct intramolecular Ullmann-type C–N formation (Scheme 1).
This unexpected and interesting result prompted us to screen the reaction conditions (Table 1). Firstly, we set out to screen the ligands in this reaction, N,N-dimethyl-ethane-1,2-diamine was found to be the best choice among the ligands (Table 1, entry 3).
| Entry | Base/equiv. | Ligandb | Solvent | Yieldc (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.5 mmol), CuI (0.1 mmol), ligand (0.2 mmol), carbonate base (1.0 mmol), and 2 mL of solvent were sealed in a pressurized process vial at 120 °C for 4 h.b Ligand: A = L-proline, B = N,N-diethylglycine, C = N,N-dimethyl-ethane-1,2-diamine, D = 1,10-phenanthroline.c Isolated yield for 2a.d The temperature of the reaction was 110 °C.e The temperature of the reaction was 100 °C. | ||||
| 1 | K2CO3/2.0 | A | DMSO | 45 |
| 2 | K2CO3/2.0 | B | DMSO | 40 |
| 3 | K2CO3/2.0 | C | DMSO | 48 |
| 4 | K2CO3/2.0 | D | DMSO | 37 |
| 5 | Cs2CO3/2.0 | C | DMSO | Trace |
| 6 | KHCO3/2.0 | C | DMSO | 55 |
| 7 | KHCO3/1.0 | C | DMSO | 68 |
| K2CO3/1.0 | ||||
| 8 | KHCO3/2.0 | C | DMSO | 74 |
| K2CO3/1.0 | ||||
| 9 | KHCO3/2.0 | C | DMSO | 74 |
| K2CO3/0.1 | ||||
| 10 | KHCO3/2.0 | C | DMF | 60 |
| K2CO3/0.1 | ||||
| 11d | KHCO3/2.0 | C | DMSO | 59 |
| K2CO3/0.1 | ||||
| 12e | KHCO3/2.0 | C | DMSO | 33 |
| K2CO3/0.1 | ||||
We then turned our attention to other C1 sources such as Cs2CO3 and KHCO3. A higher yield was obtained when KHCO3 was used (Table 1, entry 6). To our delight, the yields were improved when additional K2CO3 was used in the reaction (Table 1, entries 7 and 8). Finally, we found that the highest yield of 2a was obtained when a mixture of 2.0 equivalents of KHCO3 and 0.1 equivalent of K2CO3 was used (Table 1, entry 9). Varying the solvent from DMSO to DMF did not enhance the yield of product (Table 1, entry 10). Lower yields were obtained when the reaction temperature was reduced to 110 °C or 100 °C (Table 1, entries 11 and 12).
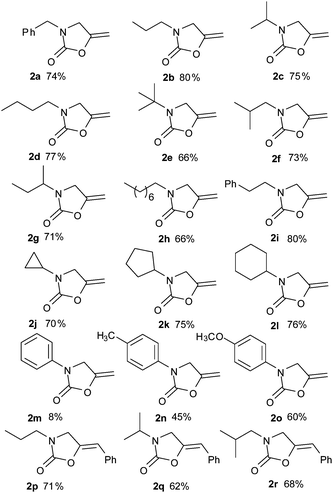
With the optimal conditions established, various N-(2-bromoallyl)amines were tested to form the corresponding oxazolidin-2-ones 2 (Table 2). As shown in Table 2, the products corresponding to the alkylamine substrates were obtained in good yields (Table 2 2a–l), and it is noteworthy that even a strained cyclopropyl substituted amine could give a corresponding ring preserved product 2j. In contrast, the reactions of arylamines led to lower yields, probably due to the poor nucleophilicity of the arylamines (Table 2 2m and n). However, the yield could be improved when an electron donating group was introduced to the benzene ring (Table 2 2o). Importantly, this process could be extended to the reactions of internal alkenyl bromides, and modest yields of the products were obtained under identical reaction conditions (Table 2 2p–r).
| a Reaction conditions: 1 (0.5 mmol), CuI (0.1 mmol), DMEDA (0.2 mmol), KHCO3 (1.0 mmol), K2CO3 (0.05 mmol), and 2 mL of DMSO were sealed in a pressurized process vial at 120 °C for 4 h. |
|---|
 |
According to the previous reports and results above,11 a proposed mechanism for this oxazolidin-2-one formation process is described in Scheme 2. When KHCO3 is heated, it produces CO2 and K2CO3. The substrate 1a reacts with CO2 in the presence of the base K2CO3 to form the carbamic acid salt X. The oxidative addition of X with the copper catalyst offers the intermediate Y. An intramolecular nucleophilic substitution in Y generates Z. The subsequent reductive elimination in Z readily affords the final product 2a and regenerates the copper species to fulfil the catalytic cycle.
To further demonstrate the applicability and efficiency of this cascade methodology, a gram-scale reaction was performed. When 1.00 g (4.4 mmol) of 1a was used as the starting material, the reaction could readily offer 0.60 g of 2a (72%).
Conclusions
In conclusion, we have developed a novel protocol for the synthesis of oxazolidin-2-ones via a cascade process of carbamic acid formation and a sequential copper-catalyzed intramolecular vinylation of carboxylic acid. The utilization of KHCO3 as the C1 source made this method efficient and convenient. Further applications of KHCO3 as a C1 source are being investigated in our laboratory.Acknowledgements
This work was supported by the Key Innovation Team of Science and Technology in Zhejiang Province (2010R50018) and Chemistry Experiment Demonstration Center of Zhejiang Province.Notes and references
- Carbon Dioxide as a Chemical Feedstock, ed. M. Aresta, Wiley-VHC, Weinheim, 2010 Search PubMed.
- Recent reviews on the use carbon dioxide for chemical transformations, see: (a) R. Zevenhoven, S. Eloneva and S. Teir, Catal. Today, 2006, 115, 73–79 CrossRef CAS PubMed; (b) T. Sakakura, J.-C. Choi and H. Yasuda, Chem. Rev., 2007, 107, 2365–2387 CrossRef CAS PubMed; (c) D. J. Darensbourg, Chem. Rev., 2007, 107, 2388–2410 CrossRef CAS PubMed; (d) M. Cokoja, C. Bruckmeier, B. Rieger, W. A. Herrmann and F. E. Kühn, Angew. Chem., Int. Ed., 2011, 50, 8510–8537 CrossRef CAS PubMed; (e) K. Huang, C.-L. Sun and Z.-J. Shi, Chem. Soc. Rev., 2011, 40, 2435–2452 RSC; (f) S. N. Riduan and Y. Zhang, Dalton Trans., 2010, 3347–3357 RSC; (g) Y. Tsuji and T. Fujihara, Chem. Commun., 2012, 48, 9956–9964 RSC.
- X.-Q. Yang, J. Wu, X.-W. Mao, T. F. Jamison and T. A. Hatton, Chem. Commun., 2014, 50, 3245–3248 RSC.
- F. Ullmann and J. Bielecki, Ber. Dtsch. Chem. Ges., 1901, 34, 2174–2185 CrossRef CAS.
- (a) G. Evano, N. Blanchard and M. Toumi, Chem. Rev., 2008, 108, 3054 CrossRef CAS PubMed; (b) F. Monnier and M. Taillefer, Angew. Chem., Int. Ed., 2009, 48, 6954–6971 CrossRef CAS PubMed; (c) S. R. Chemler, Science, 2013, 341, 624–626 CrossRef CAS PubMed.
- (a) R. K. Gujadhur, C. G. Bates and D. Venkataraman, Org. Lett., 2001, 3, 4315–4317 CrossRef CAS PubMed; (b) Y.-J. Chen and H.-H. Chen, Org. Lett., 2006, 8, 5609–5612 CrossRef CAS PubMed; (c) A. B. Naidu, E. A. Jaseer and G. Sekar, J. Org. Chem., 2009, 74, 3675–3679 CrossRef CAS PubMed; (d) D. Ma and Q. Cai, Org. Lett., 2003, 5, 3799–3802 CrossRef CAS PubMed; (e) Q. Cai, G. He and D. Ma, J. Org. Chem., 2006, 71, 5268–5273 CrossRef CAS PubMed; (f) N. Xia and M. Taillefer, Chem.–Eur. J., 2008, 14, 6037–6039 CrossRef CAS PubMed; (g) P. J. Fagan, E. Hauptman, R. Shapiro and A. Casalnuovo, J. Am. Chem. Soc., 2000, 122, 5043–5051 CrossRef CAS; (h) M. Wolter, G. Nordmann, G. E. Job and S. L. Buchwald, Org. Lett., 2002, 4, 973–976 CrossRef CAS PubMed; (i) N. Aljaar, C. C. Malakar, J. Conrad, S. Strobel, T. Schleid and U. Beifuss, J. Org. Chem., 2012, 77, 7793–7803 CrossRef CAS PubMed; (j) G. Nordmann and S. L. Buchwald, J. Am. Chem. Soc., 2003, 125, 4978–4979 CrossRef CAS PubMed; (k) Y. Fang and C. Li, J. Am. Chem. Soc., 2007, 129, 8092–8093 CrossRef CAS PubMed; (l) Y. Fang and C. Li, Chem. Commun., 2005, 3574–3576 RSC; (m) C.-Y. Chen and P. G. Dormer, J. Org. Chem., 2005, 70, 6964–6967 CrossRef CAS PubMed; (n) G. Evindar and R. A. Batey, J. Org. Chem., 2006, 71, 1802–1808 CrossRef CAS PubMed; (o) J. Iqbal, N. D. Tangellamudi, B. Dulla and S. Balasubramanian, Org. Lett., 2012, 14, 552–555 CrossRef CAS PubMed.
- C.-H. Sun, Y.-W. Fang, S. Li, Y. Zhang, Q.-W. Zhao, S.-N. Zhu and C.-Z. Li, Org. Lett., 2009, 11, 4084–4087 CrossRef CAS PubMed.
- (a) H.-W. Jin, X.-L. Xu, J.-R. Gao, J.-H. Zhong and Y.-G. Wang, Adv. Synth. Catal., 2010, 352, 347–350 CrossRef CAS; (b) H.-W. Jin, B.-W. Zhou, Z. Wu, Y. Shen and Y.-G. Wang, Tetrahedron, 2011, 67, 1178–1182 CrossRef CAS PubMed; (c) B.-W. Zhou, J.-R. Gao, D. Jiang, J.-H. Jia, Z.-P. Yang and H.-W. Jin, Synthesis, 2010, 2794–2798 CAS.
- (a) M. E. Dyen and D. Swern, Chem. Rev., 1967, 67, 197–246 CrossRef CAS; (b) D. J. Ager, I. Prakash and D. R. Schaad, Chem. Rev., 1996, 96, 835–876 CrossRef CAS PubMed; (c) D. J. Ager, I. Prakash and D. R. Schaad, Aldrichimica Acta, 1997, 30, 3–12 CAS; (d) T. A. Mukhtar and G. D. Wright, Chem. Rev., 2005, 105, 529–542 CrossRef CAS PubMed; (e) A. R. Renslo, G. W. Luehr and M. F. Gordeer, Bioorg. Med. Chem., 2006, 14, 4227–4240 CrossRef CAS PubMed.
- (a) M. Costa, G. P. Chiasoli and M. Rizzadi, Chem. Commun., 1996, 1699–1700 RSC; (b) H.-F. Jiang and J.-W. Zhao, Tetrahedron Lett., 2009, 50, 60–62 CrossRef CAS PubMed; (c) H.-F. Jiang, J.-W. Zhao and A.-Z. Wang, Synthesis, 2008, 763–769 CrossRef PubMed; (d) C.-R. Qi, J.-W. Ye, W. Zeng and H.-F. Jiang, Adv. Synth. Catal., 2010, 352, 1925–1933 CrossRef CAS; (e) H.-F. Jiang, J.-W. Ye, C.-R. Qi and L.-B. Huang, Tetrahedron Lett., 2010, 51, 928–932 CrossRef CAS PubMed; (f) J.-W. Zhao and H.-F. Jiang, Tetrahedron Lett., 2012, 53, 6999–7002 CrossRef CAS PubMed; (g) J.-W. Zhao, H.-W. Huang, C.-R. Qi and H.-F. Jiang, Eur. J. Org. Chem., 2012, 5665–5667 CrossRef CAS; (h) M. Shi and Y.-M. Shen, J. Org. Chem., 2002, 67, 16–21 CrossRef CAS PubMed; (i) Y.-L. Gu, Q.-H. Zhang, Z.-Y. Duan, J. Zhang, S.-G. Zhang and Y.-Q. Deng, J. Org. Chem., 2005, 70, 7376–7380 CrossRef CAS PubMed; (j) Z.-Z. Yang, Y.-N. Li, Y.-Y. Wei and L.-N. He, Green Chem., 2011, 13, 2351–2353 RSC; (k) Q.-W. Song, B. Yu, X.-D. Li, R. Ma, Z.-F. Diao, R.-G. Li, W. Li and L.-N. He, Green Chem., 2014, 16, 1633–1638 RSC.
- (a) D. B. Dell'Amico, F. Calderazzo, L. Labella, F. Marchetti and G. Pampaloni, Chem. Rev., 2003, 103, 3857–3897 CrossRef PubMed; (b) E. Sperotto, G. P. M. van Klink, G. van Koten and J. G. de Vries, Dalton Trans., 2010, 10338–10351 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra02304h |
| This journal is © The Royal Society of Chemistry 2014 |




