Vanadium nitride@N-doped carbon nanocomposites: tuning of pore structure and particle size through salt templating and its influence on supercapacitance in ionic liquid media†
Abstract
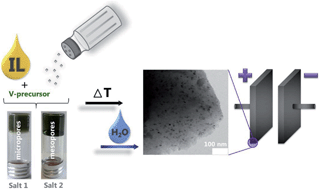
The facile one-step synthesis of composites of highly porous carbons with functional metal nitride nanoparticles with tunable surface area, pore size, pore volume and nanoparticle size is presented using simple salts as porogens. Vanadium nitride (VN) nanoparticles in high surface area nitrogen-doped carbons are made by a simple heat-treatment of mixtures consisting of a vanadium precursor (VOCl3 or NH4VO3), the ionic liquid 1-ethyl-3-methyl-imidazolium dicyanamide (Emim-dca) as solvent and the nitrogen/carbon source, and salts, i.e. cesium or zinc acetate, as porogens. The synthesis takes advantage of a homogeneous starting solution, while in situ pore generation is obtained through demixing in later stages of the reaction. As compared to other templates such as silica, the salt is easily removed with water. Furthermore, the composite properties can conveniently be controlled by the variation of the salt nature and composition of the precursor mixture. Cesium acetate as a porogen at low concentrations results in microporous materials with small VN nanoparticles with a surface area of around 1000 m2 g−1, while increasing salt amounts promote small mesopores with bigger nanoparticles and surface areas of up to 2400 m2 g−1. The utilization of zinc acetate enables the synthesis of entirely mesoporous composites with very small vanadium nitride nanoparticles and surface areas of 800 m2 g−1. Mixtures of these two salt porogens give access to independently tunable surface area, pore size, pore volume and particle size. Comparative electrochemical testing in two ionic liquid (IL) electrolytes quantifies the accessibility of the surface area in two systematic sample series and indicates optimized surface access even for large electrolytes. A variation of ion radius in similar IL-systems quantifies the accessibility of surface in the different hybrid materials. Optimal energy density of the composites in supercapacitor electrodes can only be realized in a fine balance of charge density and electronic/ionic conductivity, which is here realized by fine-tuning the structural parameters.

 Please wait while we load your content...
Please wait while we load your content...