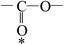
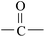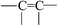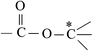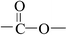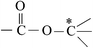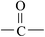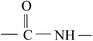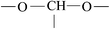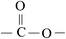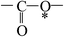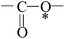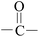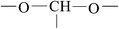Deciphering the mechanism of corona discharge treatment of BOPET film
Liping Ding,
Lu Shao and
Yongping Bai*
Harbin Institute of Technology, Harbin401#, 150001, China. E-mail: baifengbai@hit.edu.cn
First published on 28th April 2014
Abstract
The effect of corona discharge treatment on biaxially oriented polyethylene terephthalate (BOPET) film was investigated. The BOPET film and corona treated BOPET film were characterized by ATR-FTIR, XPS, SEM and AFM. It was found that many polar functional groups were generated on the surface of corona treated BOPET film and that this surface was etched after corona discharge treatment. Based on the results of the observations and analysis, a mechanism for the corona discharge treatment of BOPET film is proposed and the reactions that occur upon this treatment are put forward in detail.
Introduction
Biaxially oriented polyethylene terephthalate (BOPET) film is widely used because of its excellent and comprehensive properties,1,2 such as high transparency, thermal stability, gas barrier property, good mechanical property, flexibility, variable sizes and shapes, relative light weight, resistance to breaking, perceived high-quality image, and cost effectiveness. The production of BOPET film in the world is increasing year by year and it has become one of the indispensable polymer materials nowadays. Although having many excellent properties, BOPET film has low surface energy; it is therefore difficult to wet this film, which limits its application area. Moreover, the low surface energy greatly limits the application of BOPET film in manufacturing within processing technology fields such as hot printing, coating, and gold stamping.Corona discharge treatment2–23 has been introduced to modify the surface performance of BOPET film in recent years because this treatment is highly efficient and environmentally friendly. It has been shown to improve the film's wettability, adhesion and permeability without destroying the bulk properties. After corona discharge treatment, the surface energy of BOPET film is suitable for top-grade printing, coating and gold stamping. Therefore, understanding the mechanism for the corona discharge treatment of BOPET film is needed to provide guidance for further work. Zhi et al. showed that upon such treatment the surface of the film was etched uniformly and the surface roughness increased.24 Pandiyaraj et al. pointed out that some polar functional groups were generated on the surface of the PET film in the treatment.7 O'Hare et al. put forward that the oxygen-containing and nitrogen-containing polar functional groups were generated on the surface of the PET film and the surface morphology varied a lot.8 Gupta et al. found that the chemical composition was modified, with a physical change in surface morphology as a result of air plasma oxidation on the surface.18 Hilal et al. put forward that the plasma acting mechanism was very complex, but the main results obtained were surface cleaning, activation, crosslinking, etching, and/or (in most cases) combined effects.25 There have been other studies on the mechanism for corona discharge treatment, almost all of them suggesting that the mechanism of this treatment on BOPET film is a chemical oxidation effect14,16,26,27 that generates oxygen-containing functional groups such as carboxylic groups, anhydride groups and a small amount of hydroxyl groups. There is not yet agreement on any one specific mechanism for the corona discharge treatment of BOPET film.
For this paper, the surface performance of corona treated BOPET film was characterized by attenuated total reflectance Fourier transform infrared spectrometry (ATR-FTIR), X-ray photoelectron spectroscopy (XPS), scanning electronic microscopy (SEM) and atomic force microscopy (AFM). By researching the new functional groups generated on the surface of corona treated BOPET film, the specific reaction mechanism for the corona discharge treatment of BOPET film was systematically inferred for the first time.
Experimental
Air corona discharge treatment for BOPET film
Commercial corona treated BOPET films, each 12 μm thick, were obtained from Brückner's biaxial oriented stretching production line (Germany). The parameters of the corona discharge treatment plant are as follows: operating voltage of 16 kV, maximal input power of 26 kW, maximal output power of 20 kW, and operating frequency of 16–20 kHz. The experimental conditions of corona discharge treatment are as follows: output voltage of 8.2 kV, output power of 5.7 kW, and processing time of 1 s.ATR-FTIR for BOPET film
Absorption spectra for untreated and corona treated BOPET films were obtained by FTIR, using an attenuated total-reflection type (ATR) FT-IR spectrometer (Nicolet750).XPS for BOPET film
The chemical composition of BOPET film and corona treated BOPET film was investigated by XPS, which was performed on an X-ray photoelectron spectroscopy instrument (PerkinElmer PHI 5300, USA) using a monochromatic Mg Kalpha (1253.6 eV) photon source at an anode voltage of 10 kV, and an anode current of 20 mA. The pressure in the analyzing chamber was maintained at 10−7 Pa or lower during analysis. Data collection, processing and handling were all carried out on an Apollo Series 3500 workstation.SEM and AFM for BOPET film
Scanning electron microscopy (SEM, FEI Quanta 200, USA) and atomic force microscopy (AFM, Solver P47, Russia) were used to investigate the surface morphology of untreated BOPET film and corona treated BOPET film. SEM graphs of the film were recorded after gold-coating surface treatment and used at an accelerating voltage of 15 kV.Results and discussion
ATR-FTIR analysis
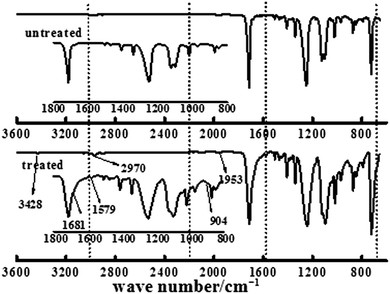
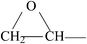
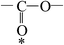
The ATR-FTIR spectra of untreated BOPET film and corona treated BOPET film are shown in Fig. 1. In order to help visualize the ATR-FTIR results, the functional groups and corresponding characteristic absorption peaks are listed in Table 1.As seen from Table 1, untreated BOPET film has the characteristic polyester absorption peaks, including those for the benzene ring, ester and methylene groups, which indicates that the basic structure of this film is in accordance with polyethylene terephthalate. The basic characteristic absorption peaks of the corona treated BOPET film are almost the same as those of the untreated BOPET film, which shows that the structures of both types of film are based on that of polyester. Nevertheless, after corona discharge treatment, the absorption peaks around 1700, 1252 and 1093 cm−1 became broad. This broadening, which involves the merging of various infrared absorption peaks, is due to very complex reactions having taken place during the corona discharge treatment. The broadening absorption peaks correspond to peroxide, carboxylic acid, carbon–carbon double bond, alcohol, phenol, aromatic ether, alkyl ethers, epoxy, polysubstituted benzene ring, amido, amino and nitrous acid ester.
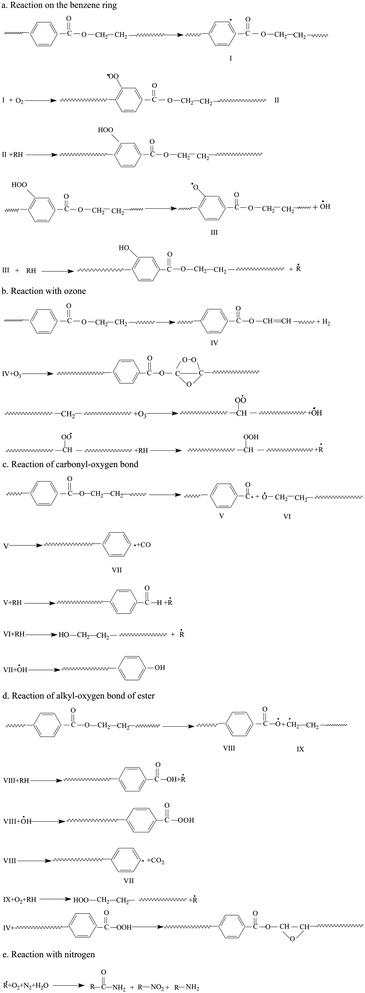
This evidence could be explained by four reactions: (a) in an oxidation reaction, the benzene ring and methylene are oxidized into oxygen-containing groups, such as phenol, ether and peroxide; (b) in a chain scission and reorientation reaction, the terephthalic acid structure is interrupted and replaced by a single benzene ring structure, or the chain reorganizes to form aldehydes (ketones), carboxylic acid and a double-bond product; (c) in a substitution reaction, the benzene ring is replaced and the polysubstituted structures are formed; and (d) in a nitridize reaction, the intermediates interact with N and O in the corona discharge treatment of the BOPET film. Nitrous acid and amine groups are incorporated into the BOPET molecular chain.
XPS analysis
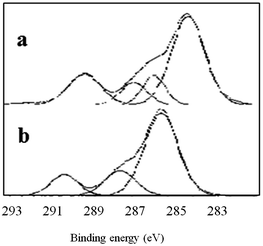
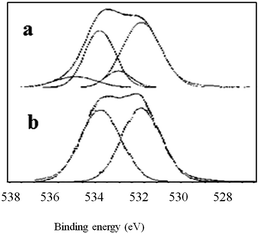
As seen in Fig. 2, the XPS C1s spectra of untreated BOPET film and corona treated BOPET film have similar peak shapes. In the binding energy range of 286 eV to 288 eV, however, the shoulders of these peaks differ between the XPS spectra of the two types of film, being relatively small and narrow in the untreated BOPET film, but larger and wider in the corona treated BOPET film. Some new groups are generated on the surface of corona treated BOPET film, such as –CONH2 (amide), –COOH (carboxylic acid), –CO (carbonyl), phenol, quinone, nitric ester, peroxides, and ozonide.10,28 The functional groups to which the C1s spectra peaks correspond are summarized in Table 2.As seen in Fig. 3, the XPS O1s spectrum of untreated BOPET film is composed of two peaks whose strength is almost equivalent, corresponding to two different oxygen atoms while the number of the atoms is nearly the same. The O1s spectrum of corona treated BOPET film is quite different from that of the BOPET film. The strength of the peak at low binding energy is decreased, while it is increased at higher binding energy, which indicates that some oxygen-containing functional groups with higher binding energy are formed, such as aldehyde, ketone, carboxylic acid, alcohol, peroxides, and ozonide. The functional groups to which the O1s spectra peaks correspond are summarized in Table 3.
A few points can be made based on Tables 2 and 3. (a) The molecular chain of untreated BOPET film has two different oxygen atoms and they are present in nearly a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, which is consistent with the ester structure of PET. The hydrocarbon content, however, is greater than the theoretical value, indicating that the molecular structure of PET has another hydrocarbon source besides the benzene ring, which may be attributed to the emitting of CO2 during polyester condensation. (b) After corona discharge treatment, the oxygen content of BOPET increases a lot. As seen from O1s/C1s, the oxygen content of corona treated BOPET film increases 7% compared with untreated BOPET film. (c) After corona discharge treatment the carbonyl or alkyl-oxygen of the ester fractures, and alcohol (phenol), aldehyde, ketone, quinone, carboxylic acid, ozonide or peroxide is generated. (d) In fact many peroxides and ozonides are generated after corona discharge treatment, which could be proved by the peak fitting of O1s at 535.42 eV, C1s at 289.74 eV and O1s at 534.44 eV. The oxygen atom of the carbonyl in the ester corresponds to the peak of O1s at 534.44 eV. But the content of O1s/C1s decreases, indicating the ester of the molecule decomposes during treatment and the content of oxygen decreases. The peak of C1s at 289.74 eV corresponds only to the carbon atom of the ester. Theoretically its content should be decreased, but its content increases by 5.53%, which indicates that the molecular chain of BOPET has a non-ester structure of high binding energy (above 289 eV). Based on combining with the actual situation of element types in the molecular chain, and excluding the possibility of the structure having a C–Cl or C–F group. Ozonide may be generated in BOPET molecular chain during treatment which provides the possibility of grafting onto the surface of BOPET. The content of ozonide cannot be ignored.
1 ratio, which is consistent with the ester structure of PET. The hydrocarbon content, however, is greater than the theoretical value, indicating that the molecular structure of PET has another hydrocarbon source besides the benzene ring, which may be attributed to the emitting of CO2 during polyester condensation. (b) After corona discharge treatment, the oxygen content of BOPET increases a lot. As seen from O1s/C1s, the oxygen content of corona treated BOPET film increases 7% compared with untreated BOPET film. (c) After corona discharge treatment the carbonyl or alkyl-oxygen of the ester fractures, and alcohol (phenol), aldehyde, ketone, quinone, carboxylic acid, ozonide or peroxide is generated. (d) In fact many peroxides and ozonides are generated after corona discharge treatment, which could be proved by the peak fitting of O1s at 535.42 eV, C1s at 289.74 eV and O1s at 534.44 eV. The oxygen atom of the carbonyl in the ester corresponds to the peak of O1s at 534.44 eV. But the content of O1s/C1s decreases, indicating the ester of the molecule decomposes during treatment and the content of oxygen decreases. The peak of C1s at 289.74 eV corresponds only to the carbon atom of the ester. Theoretically its content should be decreased, but its content increases by 5.53%, which indicates that the molecular chain of BOPET has a non-ester structure of high binding energy (above 289 eV). Based on combining with the actual situation of element types in the molecular chain, and excluding the possibility of the structure having a C–Cl or C–F group. Ozonide may be generated in BOPET molecular chain during treatment which provides the possibility of grafting onto the surface of BOPET. The content of ozonide cannot be ignored.
Based on the analysis of ATR-FTIR and XPS, a specific reaction mechanism of corona discharge treatment of BOPET is put forward. This mechanism involves many complex chemical reactions, accompanied by the emission of H2, CO and CO2.29–31 The reactions are shown in Scheme 1.
Surface morphology analysis
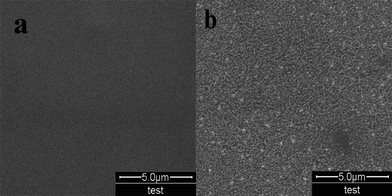
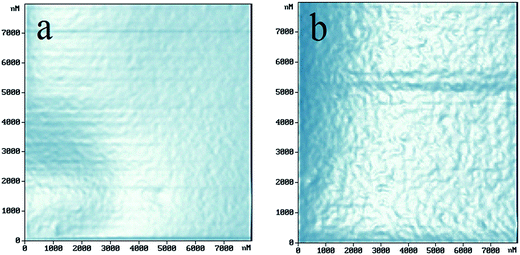
The surface morphology of BOPET film, as measured by SEM and AFM, undergoes significant changes as a result of the corona treatment. Fig. 4 and 5 are the micrographs of SEM and AFM, respectively; in each of the figures, panel a shows the untreated BOPET film and panel b shows the corona treated BOPET film. It is evident that the surface of untreated BOPET film is very smooth, but the roughness of the surface increases after corona treatment and some bright spots appear. Those bright spots are formed by some bumps made in the process of the corona treatment, which concentrate on the film's surface and appear a groove morphology formed by corona discharge treatment. It may be concluded that there is a physical change on the surface of the BOPET film during the corona treatment.Conclusion
This paper has described how the surface energy of BOPET film can be improved by corona discharge treatment to meet the needs of downstream industrial applications. The mechanism for corona discharge treatment of BOPET film was studied by analyzing ATR-FTIR spectra, XPS spectra, SEM and AFM micrographs. The results of ATR-FTIR and XPS show that new polar groups are formed on the surface of the BOPET film, which prove that the corona discharge treatment involves a chemical oxidation effect, and the specific reactions that take place during this treatment are put forward in detail. SEM and AFM micrographs show changes in the morphology of the film upon corona discharge treatment, which prove that there is a physical etching effect during this treatment. In summary, the mechanism of corona discharge treatment involves two effects: a physical etching effect in which the surface of the BOPET film is etched during the treatment and the molecular chain is interrupted; and a chemical oxidation effect, where the molecular chain of the BOPET film is subjected to chemical oxidation reactions on the basis of free radicals.References
- P. Hernandez-Muñoz, R. Catalá and R. Gavara, J. Agric. Food Chem., 1999, 47, 4370–4374 CrossRef PubMed.
- Y. Yue, J. Hou, Z. Ai, L. Yang and Q. Zhang, Plasma Sci. Technol., 2006, 8, 697 CrossRef.
- L. Ding and Y. Bai, RSC Adv., 2014, 4, 9803–9809 RSC.
- S. Weidner, G. Kühn, R. Decker, D. Roessner and J. Friedrich, J. Polym. Sci., Part A: Polym. Chem., 1998, 36, 1639–1648 CrossRef CAS.
- E. Földes, A. Tóth, E. Kálmán, E. Fekete and á. Tomasovszky-Bobák, J. Appl. Polym. Sci., 2000, 76, 1529–1541 CrossRef.
- J. Lahti, A. Savolainen, J. P. Räsänen, T. Suominen and H. Huhtinen, Polym. Eng. Sci., 2004, 44, 2052–2060 CAS.
- K. N. Pandiyaraj, V. Selvarajan, R. R. Deshmukh and M. Bousmina, Surf. Coat. Technol., 2008, 202, 4218–4226 CrossRef CAS PubMed.
- L.-A. O'Hare, J. A. Smith, S. R. Leadley, B. Parbhoo, A. J. Goodwin and J. F. Watts, Surf. Interface Anal., 2002, 33, 617–625 CrossRef CAS.
- D. K. Owens, J. Appl. Polym. Sci., 1975, 19, 3315–3326 CrossRef CAS.
- J. M. Pochan, L. J. Gerenser and J. F. Elman, Polymer, 1986, 27, 1058–1062 CrossRef CAS.
- J. Amouroux, M. Goldman and M. F. Revoil, J. Polym. Sci., Polym. Chem. Ed., 1982, 20, 1373–1387 CrossRef CAS.
- A. Laskarakis, S. Logothetidis, S. Kassavetis and E. Papaioannou, Thin Solid Films, 2008, 516, 1443–1448 CrossRef CAS PubMed.
- V. Hopfe and D. W. Sheel, Plasma Processes Polym., 2007, 4, 253–265 CrossRef CAS.
- N. A. Tabaliov and D. M. Svirachev, Appl. Surf. Sci., 2007, 253, 4242–4248 CrossRef CAS PubMed.
- R. Morent, N. De Geyter and C. Leys, Nucl. Instrum. Methods Phys. Res., Sect. B, 2008, 266, 3081–3085 CrossRef CAS PubMed.
- N. De Geyter, R. Morent and C. Leys, Nucl. Instrum. Methods Phys. Res., Sect. B, 2008, 266, 3086–3090 CrossRef CAS PubMed.
- N. L. Singh, A. Qureshi, N. Shah, A. K. Rakshit, S. Mukherjee, A. Tripathi and D. K. Avasthi, Radiat. Meas., 2005, 40, 746–749 CrossRef CAS PubMed.
- B. Gupta, J. Hilborn, C. Hollenstein, C. J. G. Plummer, R. Houriet and N. Xanthopoulos, J. Appl. Polym. Sci., 2000, 78, 1083–1091 CrossRef CAS.
- P. Esena, C. Riccardi, S. Zanini, M. Tontini, G. Poletti and F. Orsini, Surf. Coat. Technol., 2005, 200, 664–667 CrossRef CAS PubMed.
- C. Sun, D. Zhang and L. C. Wadsworth, Adv. Polym. Technol., 1999, 18, 171–180 CrossRef CAS.
- D. Zhang, Q. Sun and L. C. Wadsworth, Polym. Eng. Sci., 1998, 38, 965–970 CAS.
- K. Navaneetha Pandiyaraj, V. Selvarajan, R. R. Deshmukh and C. Gao, Vacuum, 2008, 83, 332–339 CrossRef PubMed.
- S. Yang and M. C. Gupta, Surf. Coat. Technol., 2004, 187, 172–176 CrossRef CAS PubMed.
- F. Zhi, Q. Yuchang and W. Hui, Plasma Sci. Technol., 2004, 6, 2576 CrossRef.
- N. Hilal, M. Khayet and C. J. Wright, Membrane Modification: Technology and Applications, Taylor & Francis, 2012 Search PubMed.
- A. Savolainen, Papermaking Science and Technology: Book 12: Paper and Paperboard Converting, Fapet Oy, 1998 Search PubMed.
- K. L. Mittal, Polymer Surface Modification: Relevance to Adhesion, Taylor & Francis, 2007 Search PubMed.
- J. E. Hansen, B. I. Rickett, J. H. Payer and H. Ishida, J. Polym. Sci., Part B: Polym. Phys., 1996, 34, 611–621 CrossRef CAS.
- E. Drioli and L. Giorno, Comprehensive Membrane Science and Engineering, Elsevier Science, 2010 Search PubMed.
- M. Suzuki, A. Kishida, H. Iwata and Y. Ikada, Macromolecules, 1986, 19, 1804–1808 CrossRef CAS.
- H. Iwata, A. Kishida, M. Suzuki, Y. Hata and Y. Ikada, J. Polym. Sci., Part B: Polym. Phys., 1988, 26, 3309–3322 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |