Size-controlled synthesis of uniform akaganeite nanorods and their encapsulation in alginate microbeads for arsenic removal†
Kihun Cho,
Bom Yi Shin,
Hyung Keun Park,
Bong Guen Cha and
Jaeyun Kim*
School of Chemical Engineering, Sungkyunkwan University, Suwon 440-746, Republic of Korea. E-mail: kimjaeyun@skku.edu; Tel: +82-31-290-7252
First published on 7th May 2014
Abstract
Uniform, size-controllable akaganeite nanorods were synthesized by hydrolysis of ferric ions in a two-phase system using sodium oleate as a surfactant. The akaganeite nanorods encapsulated in alginate microbeads showed quick, easy arsenic removal from highly contaminated water, indicating their potential for purification of groundwater in developing countries.
Arsenic is one of the most toxic chemicals for living organisms, and the contamination of groundwater with arsenic has raised serious threats to human health. Arsenic exposure causes various diseases including respiratory disease, abnormal skin pigmentation, hyperpigmentation, lung disease, nausea, neural injury, and kidney disease.1 Acute and chronic arsenic exposure via drinking water has been reported in many developing countries.2 Especially in Bangladesh, 20–45 million people were found to be exposed to arsenic-contaminated drinking water with a concentration above the 10 μg L−1 (10 ppb) value set by the World Health Organization (WHO) guidelines for drinking water.3 This has led to chronic arsenic exposure and associated deaths. Therefore, effective removal of arsenic from water is desired to enhance the quality of human life. Arsenic exists in the −3, 0, +3 (arsenite, As(III)) and +5 (arsenate, As(V)) oxidation states, and in the trivalanet and pentavalent oxidation states, arsenic has a toxicological structure upon environmental exposure.4 Despite the availability of many technologies for treating arsenic, their ability to remove arsenic is limited.
Main strategies for arsenic removal from water include oxidation/precipitation, coagulation/coprecipitation, ion-exchange, membrane, and sorption technologies.5 However, most of these methods suffer from slow processes, high cost, low removal efficiency, sludge generation, or difficult operation.5 Adsorption has been found to be an efficient and economical process for removing arsenic from water. Among several candidate adsorbents, akaganeite (β-FeOOH) has been investigated as a good absorbent for arsenic removal6,7 due to its high affinity toward arsenic derived from its porous tunnel structure.8 Deliyanni et al. investigated arsenic and cadmium removal from water using akaganeite nanoparticles or an akaganeite bed column.7 Usually, microsized akaganeite particles have been studied in arsenic removal, but akaganeite nanoparticles may provide better removal efficiency due to their high surface area. The synthesis of akaganeite nanoparticles has been reported, but their size-controlled synthesis is still challenging.9–11 In addition, the separation of the nanoparticles could be problematic in practical applications, such as continuous flow systems, due to their small particle size and instability. In this context, an ideal adsorbent for arsenic removal can be a porous micro-scale carrier encapsulating akaganeite nanoparticles for high mass transport and easy separation.
Alginates are naturally derived polysaccharides composed of (1-4)-linked β-D-mannuronic acid and α-L-guluronic acid monomers.12 Divalent cations cooperatively bind between the α-L-guluronic block, creating ionic cross-linking of polysaccharide chains which lead to the formation of hydrogels.13 Ionically cross-linked alginate hydrogels have been utilized as an encapsulating matrix for various types of molecules, including protein, DNA, nanoparticles, and cells,14 because alginate hydrogel has a highly porous structure which allows for efficient mass transport. In addition, alginate hydrogel can be prepared in the form of spherical microbeads, which is suitable for easy use in developing countries.15 Therefore, alginate microbeads have potential as an akaganeite carrier for arsenic removal.
Here we report the synthesis of uniform akaganeite nanorods with controllable size and their encapsulation in alginate microbeads for arsenic removal. Uniform akaganeite nanorods were prepared from the hydrolysis of ferric ions in a two-phase solution in the presence of organic surfactant. The akaganeite nanorods were encapsulated in ionically cross-linked alginate microbeads16 and tested for arsenic removal with samples containing the same level of arsenic contamination as that found in groundwater in Bangladesh in order to achieve safe arsenic levels as proposed by the WHO. Furthermore, core–shell structures composed of a core alginate microbead encapsulating magnetic nanoparticles and secondary shell alginate microbead with akaganeite nanorods embedded are proposed to prepare a magnetically separable arsenic adsorbent.
Usually akaganeite nanorods have been prepared by simple hydrolysis of ferric ions in water under heating,17 but in most of these methods, the length of the resulting akaganeite nanorods ranged from tens to hundreds of nanometers.18 The size control of akaganeite nanorods in a smaller size regime below 50 nm is still challenging. We hypothesized that the growth of akaganeite nanorods can be controlled to below 50 nm using a two-phase liquid system in the presence of surfactants. Ferric (Fe3+) ions were hydrolyzed at 60 °C in a mixture of water, ethanol, and hexane, in the presence of oleates, long alkyl chain surfactants. Yellow-brownish precipitates dispersed in the aqueous phase resulted from the reaction. The crystal structure of the as-synthesized precipitates was determined by X-ray diffraction (XRD, see SI Fig. S1†). Based on the observed peaks, the crystal structure of the precipitate was determined to be an akaganeite (β-FeOOH) structure (JCPDS Card no. 75-1549). The broad diffraction peaks of the samples indicated smaller crystallite size.
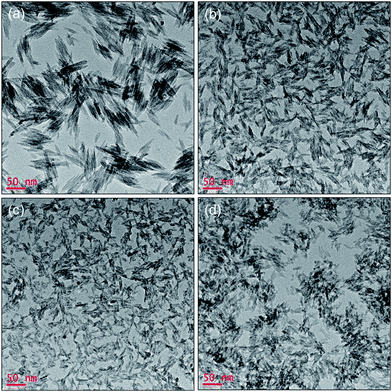
Fig. 1 shows TEM images of the precipitates obtained by varying the molar ratio between ferric ions and oleates. All precipitates were spindle-shaped nanorods. The size of the nanorods was easily controlled by the amount of oleate in the reaction mixture. Increasing the amount of oleate led to the synthesis of shorter nanorods with narrower width. When ferric ions were hydrolyzed without the addition of oleate, the largest nanorods with dimensions of 25 nm in width and 120 nm in length were produced (Fig. 1a), corresponding to an aspect ratio of around 4. When the amount of oleate was increased to the molar ratio of Fe3+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oleate = 1
oleate = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1, smaller nanorods with 13 nm width and 42 nm length were obtained (Fig. 1b). Upon further increasing the amount of oleate to the molar ratio of Fe3+
0.1, smaller nanorods with 13 nm width and 42 nm length were obtained (Fig. 1b). Upon further increasing the amount of oleate to the molar ratio of Fe3+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oleate = 1
oleate = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the dimensions of the nanorods were decreased to 6 nm in width and 23 nm in length (Fig. 1d). The nucleation of akaganeite would be enhanced by the presence of oleate, which may lead to the formation of smaller nanorods compared to the direct hydrolysis of ferric ions. Interestingly, further increasing the amount of oleates to the Fe3+
1, the dimensions of the nanorods were decreased to 6 nm in width and 23 nm in length (Fig. 1d). The nucleation of akaganeite would be enhanced by the presence of oleate, which may lead to the formation of smaller nanorods compared to the direct hydrolysis of ferric ions. Interestingly, further increasing the amount of oleates to the Fe3+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oleate ratio of 1
oleate ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 did not lead to precipitates in an aqueous phase, but to the formation of dark-brownish materials dispersed in an organic phase, which is known as an iron–oleate complex, a precursor in the synthesis of uniform magnetite nanoparticles via heat-up method.19 The nanorods dispersed in aqueous phase could only be obtained with a ratio of Fe3+ to oleate of less than 1
3 did not lead to precipitates in an aqueous phase, but to the formation of dark-brownish materials dispersed in an organic phase, which is known as an iron–oleate complex, a precursor in the synthesis of uniform magnetite nanoparticles via heat-up method.19 The nanorods dispersed in aqueous phase could only be obtained with a ratio of Fe3+ to oleate of less than 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.
3.
The oleate is a representative hydrophobic stabilizing agent for various nanoparticles to prevent aggregation in organic solvents.20 However, the akaganeite nanorods were obtained as a dispersion in water, indicating that the surface of nanorods was hydrophilic. Elemental analysis indicated that the contents of carbon and hydrogen in nanorod precipitates were negligible (C: 1.0 wt%, H: 1.1 wt%), revealing that there was almost no oleate on the surface of nanorods. Thermogravimetric analysis data also indicate that there was no significant amount of organic compounds remained with the akaganeite nanorods (TGA, see SI Fig. S2†). In the first step of 20–120 °C, 5% weight is lost due to hydrated water. In the second step of 120–280 °C, approximately 10% weight is lost by conversion from akaganeite to hematite.21 The organic phase after the synthesis of nanorods was separated and evaporated, resulting in white precipitates, oleates. Based on these observations, it is likely that the oleates participated in the formation of seeds of akaganeite at the interface of the two-phase solution and remained in organic phase after synthesis.
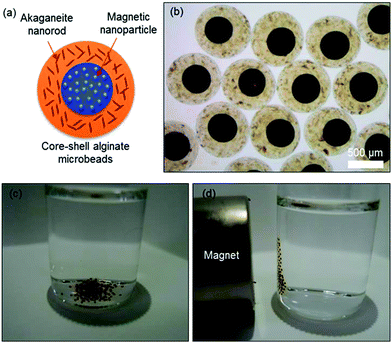
Next, the akaganeite nanorods were encapsulated in alginate microbeads for their application to arsenic removal (Scheme 1). The akaganeite nanorods dispersed in water were mixed with 2 wt% aqueous alginate solution and extruded to a divalent cationic solution via electrostatic droplet method, resulting in spherical alginate microbeads (Fig. 2). The size of the alginate microbeads was controlled by the combined conditions of applied voltage, flow rate, needle size, and dropping height. Fig. 2 shows the optical microscopic images of 400, 650, 1200, and 2000 μm sized alginate microbeads encapsulating akaganeite nanorods of 13 nm width and 42 nm length. The akaganeite nanorods were well dispersed in alginate microbeads with a slight aggregation that was likely due to the ionic strength in the alginate solution. The microbeads were stable in water for a long period of time of at least 4 weeks, which might be advantageous in practical use for arsenic removal.
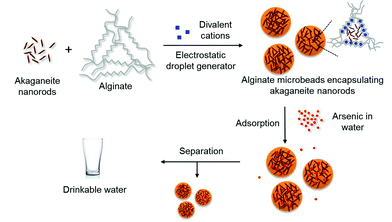 | ||
| Scheme 1 A schematic representation of the preparation of alginate microbeads encapsulated with akaganeite nanorods and their applications to arsenic removal. | ||
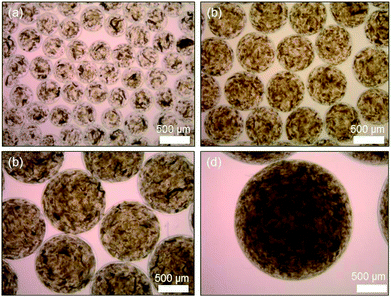 | ||
| Fig. 2 Optical microscopic images of alginate microbeads encapsulating akaganeite nanorods with average diameters of (a) 400, (b) 650, (c) 1200, and (d) 2000 μm, respectively. | ||
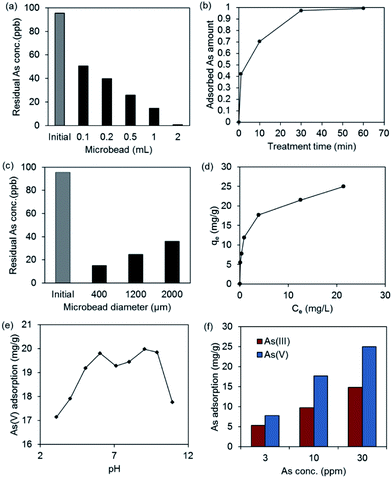
The arsenic removal from contaminated drinking water using alginate microbeads encapsulating akaganeite nanorods was tested using a 100 ppb As(V) solution mimicking the contaminated groundwater in Bangladesh, where the arsenic contamination is most severe. First, we tested different amounts of 650 μm sized alginate microbeads loaded with akaganeite nanorods. Fig. 3a shows the residual amount of arsenic after treatment of alginate microbeads with akaganeite in 100 ppb solution for 10 min. When 0.1 mL of alginate microbeads loaded with 0.92 mg of akaganeite nanorods was applied to the 100 ppb arsenic solution, the level of residual arsenic concentration was decreased to around 50 ppb, which is the national standard arsenic level of Bangladesh for drinking water. As the amount of total akaganeite used in the treatment was increased, the residual arsenic concentration was decreased. Particularly, when 2 mL of alginate microbeads encapsulating 18.3 mg of akaganeite nanorods were used in adsorption treatment, the residual arsenic concentration dramatically decreased to around 0.9 ppb, which is much lower than the 10 ppb WHO guideline. These results indicate that single treatment with the developed alginate microbeads could allow for the efficient purification of arsenic-contaminated drinking water.
To see the kinetics of arsenic adsorption in alginate microbeads encapsulating akaganeite nanorods, the residual arsenic concentration was measured over time. Fig. 3b shows the effect of treatment time on arsenic sorption onto alginate microbeads encapsulating akaganeite nanorods for an initial arsenic concentration of 100 ppb. Arsenic sorption reaches a plateau after around 30 min. The adsorption properties of arsenic on the akaganeite nanorods encapsulated in the core part of the alginate microbeads may be associated with the microbead size. Also, the negatively charged As(V) as a form of H2AsO4− or HAsO42− may feel an electrostatic repulsion, since the alginate has a negatively charged polysaccharide chain. To see the effect of microbead size on the arsenic removal, three different sizes of alginate microbeads (400, 1200, and 2000 μm in diameter) were tested in 100 ppb arsenic solution for 10 min (Fig. 3c). As expected, 400 μm sized microbeads led to the lowest residual arsenic concentration, probably due to the shorter diffusional path and high surface area through which arsenic can diffuse. The residual arsenic concentration after treatment with 400 μm sized microbeads was half that derived from 2000 μm sized microbeads. While the electrostatic droplet generator could generate around 300 μm sized alginate microbeads as the smallest size, much smaller sizes of microbeads of around 100 μm could be generated via the microfluidic technique,22 which probably increases the efficiency of arsenic removal within a shorter treatment time.
To investigate the arsenic adsorption type in alginate microbeads encapsulating akaganeite nanorods, the adsorption isotherm for As(V) solution (10 mg L−1) was collected at 25 °C under pH 7 (Fig. 3d). In order to interpret the adsorption results, the Langmuir and Freundlich isotherm models were tested. Table S1 (ESI†) shows the isotherm parameters for the Langmuir and Freundlich models obtained from linear regression. Based on the correlation coefficient, R2, a better correlation was shown by applying the Langmuir adsorption isotherm model, although both models fit the experimental data similarly.
The effect of pH on the As(V) adsorption capacity of alginate microbeads encapsulating akakaneite nanorods were also evaluated at 25 °C in 10 mg L−1 As(V) solution (Fig. 3e). It was observed that the pH value influenced the arsenic adsorption capacity of the sample. Two maximum peaks around pH 6 and 9 appeared for the adsorption of As(V) in the pH range of 3–11. This might be attributed to the change of zeta potential of the sample resulted from pH change of solution. As(V) is present as a negative ionic form of H2AsO4− below pH 7 and as HAsO42− between pH 7 and pH 11.23 The zero point of charge of akaganeite is around pH 8,24 indicating the akaganeite is positively charged below pH 8 and negatively charged above pH 8. Alginate polymer is negatively charged in most pH range examined, which resulted in repulsion of the negatively charged As(V) ions. Due to the strong electrostatic repulsion between alginate and As(V) above pH 8, the adsorption of As(V) on akaganeite nanorods would be strongly hindered. When the pH value was below 5, although the negative charge of H2AsO4− was decreased compared to HAsO42− at high pH, the high proton concentration in the solution might compete with the adsorption on positively charged akaganeite surface, which may effect on the adsorption capacity of As(V) ions. Deliyanni et al. reported that both electrostatic interaction and chemical bonding can occur between arsenic and akaganeite.25 In this study, the arsenic ions diffused into the alginate microbeads interact electrostatically with the positively charged akaganeite surface and subsequently the chemical bonding between arsenic and akaganeite could be formed.
To investigate the effect of alginate encapsulation on the arsenic adsorption, the bare and encapsulated akaganeite nanorods was tested (Fig. S3, in the ESI†). The adsorption capacity was higher in bare akaganeite nanorods compared to encapsulated one, representing that the alginate hindered the adsorption of arsenic on the akaganeite nanorods. As alginate is negatively charged, the diffusion of the negatively charged As(V) would be hindered, which presumably resulted in less adsorption of arsenic in encapsulated one.
The removal of As(III) was also compared with As(V) at pH 7 in various arsenic concentrations (Fig. 3f). It was observed that the adsorption of As(III) was lower than As(V) for all tested arsenic concentrations. This might be due to the neutral charge of As(III) as a form of H3AsO3 at pH 7, while As(V) is negatively charged. This led to weaker interaction of neutral charged As(III) than negatively charged As(V) with positively charged akaganeite.
The facile separation of the adsorbent is important in large-scale purification. We further propose core–shell type alginate microbeads composed of a core microbead encapsulating magnetic nanoparticles and a shell microbead encapsulating the core microbead and akaganeite nanorods (Fig. 4a). The microscopic image shows core–shell alginate microbeads with 400 μm sized cores and 800 μm sized shell microbeads (Fig. 4b). These microbeads could be easily separated from contaminated water using a commercial magnet (Fig. 4c and d), which may provide an alternative separation method for large-scale purification processes.
Conclusions
We have proposed a synthetic method to prepare small akaganeite nanorods less than 50 nm in length by the hydrolysis of ferric ions in a two-phase liquid system in the presence of sodium oleate. The dimensions of nanorods were easily controlled by the molar ratio of ferric ions and oleate. Using the ionically cross-linked alginate microbeads as an akaganeite carrier, arsenic removal in various conditions was tested using highly contaminated arsenic solution mimicking the groundwater in Bangladesh. It was found that the arsenic removal efficiency could be affected by the akaganeite amount, treatment time, and microbead size. Lastly, we proposed a magnetically separable alginate microbead composed of magnetite core–akaganeite shell structure. These results could be extended to macroporous membrane systems encapsulating akaganeite that can be combined with a continuous purification system.Acknowledgements
This work was supported by NRF grants (2010-0027955, 2012R1A1A1042735) funded by the National Research Foundation under the Ministry of Science, ICT & Future, Korea.Notes and references
- B. K. Mandal and K. T. Suzuki, Talanta, 2002, 58, 201–235 CrossRef CAS.
- P. Smedley and D. Kinniburgh, Appl. Geochem., 2002, 17, 517–568 CrossRef CAS.
- S. V. Flanagan, R. B. Johnston and Y. Zheng, Bull. W. H. O., 2012, 90, 839–846 CrossRef PubMed.
- C. Jain and I. Ali, Water Res., 2000, 34, 4304–4312 CrossRef CAS.
- D. Mohan and C. U. Pittman Jr, J. Hazard. Mater., 2007, 142, 1–53 CrossRef CAS PubMed.
- E. Deliyanni, D. Bakoyannakis, A. Zouboulis and K. Matis, Chemosphere, 2003, 50, 155–163 CrossRef CAS.
- E. Deliyanni, D. Bakoyannakis, A. Zouboulis and E. Peleka, Sep. Sci. Technol., 2003, 38, 3967–3981 CrossRef CAS PubMed.
- J. H. Johnston and N. E. Logan, J. Chem. Soc., Dalton Trans., 1979, 22, 13–16 RSC.
- Z. Yuan and B. Su, Chem. Phys. Lett., 2003, 381, 710–714 CrossRef CAS PubMed.
- J. Cai, J. Liu, Z. Gao, A. Navrotsky and S. L. Suib, Chem. Mater., 2001, 13, 4595–4602 CrossRef CAS.
- D. Bakoyannakis, E. Deliyanni, A. Zouboulis, K. Matis, L. Nalbandian and T. Kehagias, Microporous Mesoporous Mater., 2003, 59, 35–42 CrossRef CAS.
- A. Martinsen, G. Skjåk-Bræk and O. Smidsrød, Biotechnol. Bioeng., 1989, 33, 79–89 CrossRef CAS PubMed.
- M. S. Shoichet, R. H. Li, M. L. White and S. R. Winn, Biotechnol. Bioeng., 1996, 50, 374–381 CrossRef CAS.
- A. D. Augst, H. J. Kong and D. J. Mooney, Macromol. Biosci., 2006, 6, 623–633 CrossRef CAS PubMed.
- A. I. Zouboulis and I. A. Katsoyiannis, Ind. Eng. Chem. Res., 2002, 41, 6149–6155 CrossRef CAS.
- V. A. Nedović, B. Obradović, I. Leskošek-Čukalović, O. Trifunović, R. Pešić and B. Bugarski, Process Biochem., 2001, 37, 17–22 CrossRef.
- Y. Sahoo, A. Goodarzi, M. T. Swihart, T. Y. Ohulchanskyy, N. Kaur, E. P. Furlani and P. N. Prasad, J. Phys. Chem. B, 2005, 109, 3879–3885 CrossRef CAS PubMed.
- J. Zhao, W. Lin, Q. Chang, W. Li and Y. Lai, Environ. Technol. Rev., 2012, 1, 114–126 CrossRef CAS.
- J. Park, K. An, Y. Hwang, J. Park, H. Noh, J. Kim, J. Park, N. Hwang and T. Hyeon, Nat. Mater., 2004, 3, 891–895 CrossRef CAS PubMed.
- B. P. Binks, Curr. Opin. Colloid Interface Sci., 2002, 7, 21–41 CrossRef CAS.
- K. Ståhl, K. Nielsen, J. Jiang, B. Lebech, J. C. Hanson, P. Norby and J. van Lanschot, Corros. Sci., 2003, 45, 2563–2575 CrossRef.
- K. Liu, H. Ding, J. Liu, Y. Chen and X. Zhao, Langmuir, 2006, 22, 9453–9457 CrossRef PubMed.
- T. L. Xu, Y. Cai and K. E. O'Shea, Environ. Sci. Technol., 2007, 41, 5471–5477 CrossRef CAS.
- R. Chitrakar, S. Tezuka, A. Sonoda, K. Sakane, K. Ooi and T. H. Hirotsu, J. Colloid Interface Sci., 2006, 298, 602–608 CrossRef CAS PubMed.
- E. A. Deliyanni, D. N. Bakoyannakis, A. I. Zouboulis and K. A. Matis, Chemosphere, 2003, 50, 155–163 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and relevant figures. See DOI: 10.1039/c4ra01998a |
| This journal is © The Royal Society of Chemistry 2014 |