Preparation and characterization of MTiX for the catalytic oxidation of cyclohexane†
Abstract
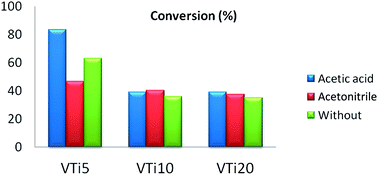
MTiX (M = Cr and V) samples, with 0, 5, 10 and 20 atomic percent, were synthesised by a sol–gel method and calcined at 400 °C in air. The XRD analysis results indicated that the materials presented a crystalline structure with the presence of TiO2 anatase forms. BET analysis showed that the surface area varied with the variation of metal content. EDX analysis showed a heterogeneous distribution of samples. The results of pyridine adsorption followed by FT-IR indicated that Brønsted and Lewis acid sites are present on the surface of catalysts. Liquid phase oxidation of cyclohexane was carried out under milder reaction conditions over MTiX catalysts using tert-butyl hydroperoxide (TBHP) as oxidant, acetic acid and acetonitrile as solvents. The results indicated that conversion depends on metal content and solvent polarity. Chromium is selective for cyclohexanol. The best result was obtained with VTi20 with 35% of conversion and 92% of olone selectivity.

 Please wait while we load your content...
Please wait while we load your content...