Chemical decomposition of epoxy resin in near-critical water by an acid–base catalytic method
Yuyan Liu*a,
Hongjun Kanga,
Xianyun Gongab,
Liqin Jianga,
Yuting Liua and
Sonquan Wu*a
aSchool of Chemical Engineering and Technology, Harbin Institute of Technology, Harbin 150001, P. R. China. E-mail: liuyy@hit.edu.cn; wusongquan@hit.edu.cn; Fax: +86-0451-86413711; Tel: +86-0451-86413711
bSchool of Science, Department of Chemistry, Harbin University, Harbin 150086, P. R. China
First published on 24th April 2014
Abstract
Chemical decomposition of an epoxy resin (E-51) cured with methyl tetrahydro phthalic anhydride (MeTHPA) in near-critical water conditions was investigated under different concentrations of acid–base catalyst. The optimal catalytic concentration of KOH catalyst ranged from 0.5 mol L−1 to 1.0 mol L−1, while that of H2SO4 catalyst was 0.4 mol L−1. Meanwhile, the decomposition ratio of the E-51/MeTHPA system could reach up to 97.7–100%. In addition, FT-IR results of the solid residue of the E-51/MeTHPA system before and after near-critical water treatment showed that the changes of the molecular structure were mainly reflected in the changes of the relative contents of mixed ether bonds, cross-linked bonds and other functional groups. The decomposition products in the acetone phase were identified by GC-MS. The results suggested that the main compositions and relative peak areas of the decomposition products varied with the change of the concentration of acid–base catalyst. Finally, a possible decomposition reaction mechanism was proposed for the E-51/MeTHPA system.
1. Introduction
Epoxy resin, considered as a typical thermosetting plastic, is one of the most versatile polymers today. It exhibits some extraordinary properties such as strong adhesive force, good dielectric properties and high stability. Therefore, it has been widely applied in adhesive, printed circuit boards and advanced composite materials.1–3 However, when these epoxy resin matrix materials reach their useful lives, the disposing of these waste materials becomes a serous issue. Both landfill and incineration disposal are not optimal methods due to environmental issues. At the same time, it is usually difficult to recycle epoxy resin because of its three-dimensional network structure. Therefore, an efficient recycling method for epoxy resin should be friendly to the environment and obtain some useful chemicals.So far, the known recycling technologies for epoxy resin mainly include mechanical recycling,4 thermal recycling5–7 and chemical recycling.8 Among them, the chemical recycling is a new and promising route to transform polymer matrices of high molecular weight into low molecular weight molecules.9,10 In recent years, supercritical water (SCW, T = 374 °C, P = 22.1 MPa) and near-critical water (NCW, temperature at 250–350 °C) methods, which belong to chemical recycling methods, have been considered novel and effective methods to degrade epoxy resin due to the fact that water is nontoxic, cost-effective, readily available and is classed as a green media.11–13 In previous studies, P. H. Raul et al. succeed in recycling carbon fiber composites under subcritical and supercritical conditions using alcohols as reactive-extraction media.14 Y. P. Bai et al. recycled carbon fibers from CFRP in supercritical water in the presence of oxygen and found that oxygen could promote the decomposition of epoxy resin.15 J. Liu et al. recycled carbon fiber reinforced epoxy resin composites in subcritical water with the addition of phenol and KOH and discovered that the combination of KOH and phenol could promote the decomposition efficiency.16
In this study, near-critical water (NCW) method was applied to investigate the decomposition of E-51/MeTHPA system. The effects of different catalyst types and concentrations on the decomposition ratio and decomposition products of E-51/MeTHPA system were investigated to obtain the optimal decomposition condition. Meanwhile, a possible decomposition reaction mechanism was proposed for E-51/MeTHPA system.
2. Experimental
2.1 Materials
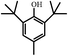
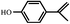
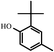
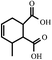
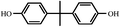
Commercial diglycidyl ether of bisphenol A type epoxy resin (E-51) was supplied by Wuxi Resin Factory, China. The curing agent, methyl tetrahydro phthalic anhydride (MeTHPA) and the accelerant, benzyl dimethylamine (BDMA), were purchased from business agents. The chemical structures of E-51 and MeTHPA are illustrated in Fig. 1.2.2 Preparation of E-51/MeTHPA sample
The epoxy resin (E-51), the curing agent (MeTHPA) and accelerant (BDMA) were mixed according to a mass ratio of 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 84.7
84.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and then stirred constantly with a glass rod until a homogeneous mixture was obtained. The above blend was injected into the stainless steel mold before reaching the gel time and cured at 100 °C for 2 h and then heated to 150 °C for another 5 h. Finally, the cured epoxy resin was cut into cubic samples with size of 20 mm × 20 mm × 2 mm.
1 and then stirred constantly with a glass rod until a homogeneous mixture was obtained. The above blend was injected into the stainless steel mold before reaching the gel time and cured at 100 °C for 2 h and then heated to 150 °C for another 5 h. Finally, the cured epoxy resin was cut into cubic samples with size of 20 mm × 20 mm × 2 mm.
2.3 Decomposition of E-51/MeTHPA sample
The decomposition reaction was conducted in batch autoclave. The apparatus is mainly composed of a removable stainless steel reactor (100 mL) connected to a pressure gauge, a temperature controller and a salt bath furnace to heat the reactor. The decomposition temperature and pressure could be adjusted. In the experiment, 1 g of sample and 10 mL distilled water were employed in the reactor and the feedstock ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (g mL−1). The reactions were carried out at 270 °C for 45 min in a near-critical condition. When the decomposition reaction was finished, the removable reactor was put into cold water and cooled to room temperature. The aqueous phase decomposition products were collected. The solid residues were immersed in acetone for 24 h, washed 3 times with distilled water and dried at 100 °C for 24 h in a vacuum oven. Finally, the collected acetone immersing liquid was analyzed by GC-MS and the dried solid residues were weighted to calculate the decomposition ratio using the following formula (1). The decomposition ratio was averaged with each experiment repeated three times.
10 (g mL−1). The reactions were carried out at 270 °C for 45 min in a near-critical condition. When the decomposition reaction was finished, the removable reactor was put into cold water and cooled to room temperature. The aqueous phase decomposition products were collected. The solid residues were immersed in acetone for 24 h, washed 3 times with distilled water and dried at 100 °C for 24 h in a vacuum oven. Finally, the collected acetone immersing liquid was analyzed by GC-MS and the dried solid residues were weighted to calculate the decomposition ratio using the following formula (1). The decomposition ratio was averaged with each experiment repeated three times.
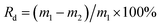 | (1) |
2.4 Characterization
The cured degree of E-51/MeTHPA was measured by DSC (TA Q200, USA). The sample was heated from 25 °C to 250 °C at the heating rate of 10 °C min−1 under nitrogen atmosphere. Thermo-gravimetric test was carried out in the air atmosphere by TGA (ZRP-2Y) to study the thermal oxidation decomposition of the E-51/MeTHPA system. The changes of the molecular structures of epoxy resin before and after treatment and the location of cleavage of the chemical bonds in the reaction path-way were analyzed by using Fourier transform infrared spectroscopy (FT-IR) (VERTEX 70). In order to determine the composition and distribution of liquid products in acetone phase, GC-MS (HP-6890GC) equipped with HP-5MS elastic quartz capillary column (30 m × 0.25 m × 0.25 μm) was used. The operating conditions were as follows: injection temperature was 280 °C; the high purity helium was used as carrier gas at the flow rate of 1.0 mL min−1; column temperature was form 50 °C to 280 °C; ion source temperature was 230 °C. The warming parameters were as follows: heating rate was 10 °C min−1 in the range of 50–180 °C; heating rate was 20 °C min−1 in the range of 180–280 °C. The scanning range of MS was 0–500 amu and electron bombardment source was 70 eV.3. Results and discussion
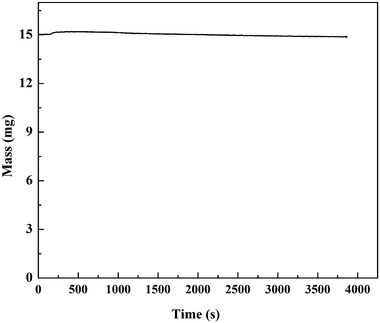
The DSC result indicated that the E-51/MeTHPA system was cured completely because the post curing peak did not appear in the DCS curve.3.1 The thermo-gravimetric analysis of E-51/MeTHPA system
In order to study the influence of the thermal oxidation decomposition on the E-51/MeTHPA system, thermo-gravimetric test was carried out in the air atmosphere. Fig. 2 shows the thermo-gravimetric curve of the E-51/MeTHPA system at 270 °C for 45 min. From the Fig. 2, it could be clearly found that the E-51/MeTHPA system began to loss weight at about 16 min and the descent part of the curve was smooth, indicating that the weight loss of the E-51/MeTHPA system was quite little in the process of heat treatment for 45 min and the weight loss was about 3%.3.2 Effect of acid–base catalysts on the decomposition ratio
In order to obtain the optimal catalytic decomposition condition, the effect of concentration of acid–base catalyst on the decomposition ratio of the E-51/MeTHPA system was investigated under the following conditions: a reaction time of 45 min, a reaction temperature of 270 °C and catalyst concentration from 0 mol L−1 to 1.0 mol L−1. Fig. 3 illustrates the effect of the concentration of H2SO4 catalyst on the decomposition ratio of the E-51/MeTHPA system in near-critical water. As can be seen from Fig. 3, when the concentration of H2SO4 catalyst was less than 0.4 mol L−1, with increasing concentration of H2SO4 catalyst, the decomposition ratio increased firstly, then experienced a little decrease, and finally continued to increase. When the concentration of H2SO4 catalyst was 0.4 mol L−1, the decomposition ratio reached 97.7 wt%. Surprisingly, the decomposition ratio decreased to 76 wt% with the further increasing concentration of H2SO4 catalyst. Therefore, the appropriate concentration of H2SO4 catalyst was crucial to the decomposition of E-51/MeTHPA system in near-critical water. From the above analysis, it indicated that the optimal catalytic concentration of H2SO4 catalyst was 0.4 mol L−1. | ||
| Fig. 3 The curve of decomposition ratio of the E-51/MeTHPA cured system versus the concentration of H2SO4 catalyst. Reaction conditions: T = 270 °C, t = 45 min, feedstock = 1/10 (g mL−1). | ||
Fig. 4 shows the influence of the concentration of KOH catalyst on the decomposition ratio of E-51/MeTHPA system in near-critical water. As can be seen from Fig. 4, the decomposition ratio of the E-51/MeTHPA system was almost 41.1 wt% without adding the KOH catalyst. When the concentration of KOH catalyst was 0.05 mol L−1, the decomposition ratio of the resin system significantly increased to 85 wt%. With the increasing concentration of KOH catalyst, the decomposition ratio continued to increase in general. When the concentration of KOH catalyst increased to 0.5 mol L−1, the E-51/MeTHPA system was decomposed completely and the decomposition ratio nearly reached 100 wt%. From the above analysis, the optimal catalytic concentration of KOH catalyst was in the range from 0.5 mol L−1 to 1.0 mol L−1.
 | ||
| Fig. 4 The curve of decomposition ratio of the E-51/MeTHPA cured system versus the concentration of KOH catalyst. Reaction conditions: T = 270 °C, t = 45 min, feedstock = 1/10 (g mL−1). | ||
Compared the effects of acid catalyst (H2SO4) and base catalyst (KOH) on the decomposition ratio of the E-51/MeTHPA system, it could be concluded that they all could improve the decomposition ratio of the resin system, and the decomposition ratio could reach 97.7–100 wt%. This could be explained as the following: the decomposition ratio of the resin system was determined by the concentration of hydroxyl or hydronium ions in near-critical water condition and the outstanding effect of KOH and H2SO4 catalysts on the decomposition ratio of the E-51/MeTHPA system mainly resulted from a promotion effect of the concentration of hydroxyl or hydronium ions.
3.3 The analysis of decomposition residues
To infer the decomposition reaction mechanism of the E-51/MeTHPA system, it is needed to identify the fracture location of the chemical bonds. The structures and main functional groups of the E-51/MeTHPA system before and after near-critical water treatment were investigated by FT-IR. The FT-IR spectra of original resin and catalytic decomposition residues are shown in Fig. 5. Compared (a) and (b) spectra, it could be clearly found that the intensity of hydroxyl characteristic absorption peak at 3454 cm−1 of the H2SO4 catalytic decomposition residue increased. It could be attributed to the increase of phenolic hydroxyl, generated from homolysis or hydrolysis of ether bond in the main chain during the decomposition reaction. In addition, the intensity of carbonyl characteristic absorption peak at 1741 cm−1 also increased, indicating that the crosslink bond of the E-51/MeTHPA system was cleaved to generate the aromatic acid and anhydride molecule. Meanwhile, the carbon–hydrogen out-of-plane bending vibration peak of benzene ring in the aromatic acid and anhydride molecule had a certain displacement, which illustrated that the breakage of main chain was accompanied by rearrangement reactions and the substituted position of phenyl changed during the degradation process of the resin. Compared (a) and (c) spectra, it could be found the similar changes for the KOH catalytic decomposition residues (Fig. 5). | ||
| Fig. 5 The FT-IR spectra of original resin and catalytic decomposition residues: (a) original resin; (b) H2SO4 catalytic decomposition residue and (c) KOH catalytic decomposition residue. | ||
From the above analysis, we could find that the changes of the structure of solid residue were mainly reflected in the content change of ether, ester bond and other functional groups in catalytic decomposition process. Carbon–carbon bond and ether bond in the main chain of epoxy as well as the crosslink bond of the E-51/MeTHPA system were broken under the action of hydrolysis, and then combined with the active hydrogen or hydroxide ions in the system to form substances with stable structure.
3.4 The analysis of liquid decomposition products
In order to understand the organic compositions of the liquid decomposition products in acid–base catalytic decomposition process, the liquid decomposition products in acetone phase were analyzed by GC/MS. Table 1 presents the major components of the liquid catalytic decomposition products in near-critical water condition with 0.2 mol L−1 KOH catalyst. From Table 1, the major components of the liquid decomposition products mainly included phenol, bisphenol A, p-isopropenyl phenol and 2,6-di-tert-butyl-4-methylphenol (BHT), whose relative peak area were 48.09%, 6.97%, 7.68% and 37.27% respectively. It was worth mentioning that the relative peak area of phenol almost reached to 50%, indicating that the E-51/MeTHPA system did not undergo the oxidation–reduction reaction or any other side reactions under this reaction condition. Anhydride related products were not detected in an alkaline environment of low concentration, indicating that the stability of the ether bond reduced, which caused the large-scale fracture of mixed ether bonds in the main chain, and the hydrolysis of ester bonds was inhibited. In addition, the relative peak area of bisphenol A was relatively high, implying that it was conducive to recover bisphenol A and phenol related derivatives when the concentration of KOH catalyst was low.The main components the liquid decomposition in near-critical water condition with 0.5 mol L−1 KOH catalyst were shown in Table 2. From the Table 2, it could be found that the major components of the liquid decomposition products were phenol, benzyl alcohol, p-isopropylphenol, p-isopropenyl phenol, bisphenol A and 2,6-di-tert-butyl-4-methylphenol (BHT), whose relative peak area were 64.69%, 2.95%, 7.90%, 3.89%, 0.85% and 7.13% respectively. Meanwhile, the majority of decomposition products were phenolic compounds and anhydride related products were still not detected in an alkaline environment of high concentration. Compared to Table 1, it could be found that the relative peak area of phenol compound in the liquid decomposition products increased from 48.09% to 64.69%, which could be explained that the mixed ether bonds in the main chain generated the large-scale fracture and the inhibition degree of hydrolysis of the ester bond strengthened. The relative peak area of bisphenol A compound decreased from 6.97% to 0.85%, which might be attributed to the decrease of stability of bisphenol A, which could be transformed into phenol and p-isopropenyl phenol with the increasing of concentration of KOH catalyst. Therefore, the change of the concentration of KOH catalyst had selectivity to the compositions of the liquid decomposition products.
Likewise, the major components of the liquid catalytic decomposition products in near-critical water condition with different concentration of H2SO4 catalyst were investigated by GC/MS. Table 3 presents the major components of the liquid catalytic decomposition products in near-critical water condition with 0.1 mol L−1 H2SO4 catalyst. The major compounds the major components of the liquid decomposition products were phenolic compounds, anhydrides and a small quantity of alcohols, whose relative peak area were 43.62%, 18.69% and 7.44% respectively. This result indicated that H2SO4 played an important part in promoting the hydrolysis of ether bond in the main chain and cross-linked bonds between resin and curing agent. Hydrolysis of ether bond was still dominant relative to that of cross-linked ester bond.
Table 4 shows the major components of the liquid catalytic decomposition products in near-critical water condition with 0.4 mol L−1 H2SO4 catalyst. It could be found that the major components of the liquid decomposition products were still phenolic compounds, anhydrides and a small quantity of alcohols, whose relative peak area were 32.1%, 33.88% and 2.78% respectively. Compared with Table 3, the relative peak area of phenol, anhydrides and aromatic dicarboxylic acid increased significantly, implying that the increasing of H2SO4 catalyst concentration was advantageous to the hydrolysis of the cross-linked bonds between the resin and curing agent. Through the above analysis, it could be concluded that the change of the concentration of H2SO4 catalyst had selectivity to the way of bond breaking of the resin system.
In summary, it could be concluded that KOH and H2SO4 catalysts could promote the fracture of mixed ether and ester bonds in different extent and change the composition of the liquid decomposition products. When KOH was used as catalyst, the content of phenolic compounds was higher, implying that KOH catalyst restrained the hydrolysis of ester bonds and had a stronger role in promoting the hydrolysis of ether bonds. When H2SO4 was used as catalyst, the breaking proportion of ether bonds and ester bonds were in equal.
3.5 Mechanism of the decomposition reaction of E-51/MeTHPA system
According to FT-IR and GC-MS analysis, the type and concentration of the catalyst had a great influence on the compositions of the decomposition products of the E-51/MeTHPA system, implying that the catalyst changed the reaction pathway in different degree during the decomposition process.Near-critical water is considered to be an excellent reaction medium for organic chemical reactions. The decomposition reaction mechanism of the E-51/MeTHPA system in the near-critical water is proposed. Based on the previous analysis, the hydrolysis of mixed ether bonds and cross-linked bonds of the E-51/MeTHPA system was the main way of bond breaking. Scheme 1 illustrates possible mechanisms of the decomposition reaction of the E-51/MeTHPA system with different acid–base catalysts.
 | ||
| Scheme 1 Possible decomposition mechanism of the E-51/MeTHPA cured system in near-critical water with acid–base catalysts. | ||
From the results of GC-MS, phenol and phenol derivatives in the decomposition products were in high proportion when KOH was used as catalyst. On the above basis, KOH catalyst could accelerate the breakage of ether bond of the E-51/MeTHPA system, while the breakage of ester bond was restrained. Therefore, the decomposition reaction could be followed the path 2, 3 or 2, 3, 4 in Scheme 1, where the path 2, 3 was the main path. First, the hydronium ions attacked the ether bond in the main chain and decomposed them into bisphenol A monomer or other oligomers. After the bisphenol A could be further decomposed to phenol, p-isopropylphenol, p-isopropenyl phenol and other substances. However, when H2SO4 was used as the catalyst, the proportion of phenolic compounds was equivalent to that of anhydride compounds. H2SO4 catalyst had the same promotion effect on the breakage of ether bond and ester bond. Therefore, the decomposition reaction could be mainly followed the route 1, 2, 3, 4 in Scheme 1. First, the hydronium ions attacked the ether bond ester bond simultaneously in the main chain and decomposed them into bisphenol A, 3-methyl-4-cyclohexene-1,2-dicarboxylic acid, methyl tetrahydro phthalic anhydride and other oligomers, which could be followed the path 1, 2 in the Scheme 1. Then, the bisphenol A could be decomposed to phenol and the phenol could further undergo rearrangement reactions with some small molecules (i.e. propylene and methyl) to form the 2,6-di-tert-butyl-p-cresol and 2-tert-butyl-p-cresol, which could be followed the path 3, 4 in the Scheme 1.
4. The quantitative comparison with current methods
The quantitative comparison data with current treatment methods of epoxy resin have been shown in Table 5, including pyrolysis treatment, conventional acid–base catalytic treatment, microwave treatment and new near-critical water treatment (super-critical fluids). From the Table 5, it could be found that the liquid yield and purity of phenol increased obviously when the CaCO3 catalyst was added into the pyrolysis system, indicating that the decomposition ratio increased and the compositions of decomposition products had been changed with the addition of the CaCO3 catalyst.17 When the nitric acid was applied in decomposing the epoxy resin, the required time for complete decomposition of the epoxy resin was 100 h and the phenol and phenol derivatives could not be detected.18 For the microwave (HNO3) treatment system, it took a total of 75 min for the epoxy resin to be completely dissolved in the nitric acid and the decomposition product was 1,3-di(ethyl ester)-5-(diethyl amino)-2-hydroxybenzene.19 However, for the new decomposition method of supercritical isopropanol (SCP), the decomposition ratio could be reach about 92% and the decomposition products were phenol and phenol derivatives. At the same time, the purity of phenol obtained with no KOH catalyst was higher than that obtained with 0.8 wt% KOH catalyst.20| Methods | Temperature (°C) | Time (min) | Liquid yield (%) | Purity of phenol (%) | Source |
|---|---|---|---|---|---|
| Pyrolysis | 400 | 60 | 35.1 | 2.4 | Sato et al.17 |
| Pyrolysis (CaCO3) | 61.9 | 9.4 | |||
| Nitric acid | 80 | 6000 | 100 | — | Dong et al.18 |
| Microwave (HNO3) | 120 | 75 | 100 | — | Bolasodun et al.19 |
| SCP (no KOH) | 293 | 20 | 92.12 | 30.76 | Jiang et al.20 |
| SCP (0.8% KOH) | 264–282 | 92.65 | 10.53 | ||
| NCW (0.4 M KOH) | 270 | 45 | 100 | 64.69 | This work |
| NCW (0.5 M H2SO4) | 97.7 | 18.67 |
In the present study, the near-critical water (NSW) was used for the decomposition of the E-51/MeTHPA system. The differences of the reaction condition, the acid–base catalyst, liquid yield and purity of phenol among the pyrolysis, nitric acid catalytic, microwave treatment, super-critical isopropanol (SCP) and near-critical water treatment (NSW) could be shown in Table 5. The advantages of the near-critical water treatment could be demonstrated as follows. First, the temperature applied in near-critical water treatment (270 °C) was much lower than the pyrolysis (400 °C) and the treatment time was shortened obviously, compared to the nitric acid treatment, so the energy consumption could be reduced. Second, high purity of phenol (64.69%) could be obtained by using near-critical water treatment with 0.5 mol L−1 KOH catalyst, which was much higher than that of the pyrolysis and SCP. Therefore, the high purity of phenol could be further purified to synthesize some other compounds (i.e. phenolic resin). Finally, during the treatment process, the reaction media was water, which is classed as a green media, due to its nontoxic, cost-effective, readily available.
5. Conclusions
(1) KOH and H2SO4 catalysts could promote the decomposition ratio of the E-51/MeTHPA system and with the concentration of KOH or H2SO4 catalyst increasing, the decomposition ratio increased in general. The optimal catalytic concentration of KOH catalyst ranged from 0.5 mol L−1 to 1.0 mol L−1, while that of H2SO4 catalyst was 0.4 mol L−1. Meanwhile, the decomposition ratio of the E-51/MeTHPA system could reach up to 97.7–100% at 270 °C for 45 min in near-critical water condition with the optimal concentration of acid–base catalysts.(2) FT-IR results of the solid residue of the E-51/MeTHPA system before and after near-critical water treatment showed that the changes of the molecular structure were mainly reflected in the changes of relative contents of mixed ether bonds, cross-linked bonds and other functional groups. Mixed ether bonds in the main chain and cross-linking of the E-51/MeTHPA system were broken under the action of hydrolysis, and then combined with the active hydrogen and hydroxide ions in the system to form substances with stable structure.
(3) GC-MS was used to investigate the composition of liquid decomposition products in near-critical water condition with acid–base catalyst. When KOH was used as catalyst, phenol and phenol derivatives in the decomposition products were in high proportion. However, when H2SO4 was used as the catalyst, the major compositions of liquid decomposition products mainly included phenolic compounds and anhydride compounds.
(4) KOH and H2SO4 had different influences on the way of decomposition of the E-51/MeTHPA system. KOH catalyst could accelerate the breakage of ether bond of the E-51/MeTHPA system, while the breakage of ester bond was restrained. However, H2SO4 catalyst had the same promotion effect on the breakage of ether bond and ester bond.
Acknowledgements
This work was financially supported by the National Science Foundation of China (NSFC-50973023) and Program for New Century Excellent Talents in University (NCET-09-0060).References
- I. Yarovsky and E. Evans, Polymer, 2002, 43, 963 CrossRef CAS.
- H. Ren, J. Z. Sun, B. J. Wu and Q. Y. Zhou, Polymer, 2006, 47, 8309 CrossRef CAS PubMed.
- T. Abraham, Chem. Eng. World., 1994, 29, 31 CAS.
- C. E. Kouparitsas, C. N. Kartalis, P. C. Vareilidis, C. J. Tsenoglou and C. D. Papaspyrides, Polym. Compos., 2002, 23, 682 CrossRef CAS.
- S. J. Pickering, Composites, Part A, 2006, 37, 1206 CrossRef PubMed.
- T. Iwaya, S. Tokuno, M. Sasaki, M. Goto and K. Shibata, J. Mater. Sci., 2008, 43, 2452 CrossRef CAS PubMed.
- N. Grassie, Polym. Degrad. Stab., 1989, 14, 125 CrossRef.
- W. R. Dang, M. Kubouchi, H. Sembokuya and K. Tsuda, Polymer, 2008, 46, 1905 CrossRef PubMed.
- F. Sasse and G. Emig, Chem. Eng. Technol., 1998, 21, 780 CrossRef.
- G. Grause, M. Furusawa, A. Okuwaki and T. Yoshioka, Chemosphere, 2008, 71, 875 CrossRef PubMed.
- M. Goto, J. Supercrit. Fluids, 2009, 47, 500 CrossRef CAS PubMed.
- C. Fromonteil, P. Bardelle and F. Cansell, Ind. Eng. Chem. Res., 2000, 39, 922 CrossRef CAS.
- K. Arai, R. L. Smith and T. M. Aida, J. Supercrit. Fluids, 2009, 47, 628 CrossRef CAS PubMed.
- R. Piñero-Hernanz, J. García-Serna, C. Dodds, J. Hyde, M. Poliakoff and M. J. Cocero, et al., J. Supercrit. Fluids, 2008, 46, 83 CrossRef PubMed.
- Y. P. Bai, Z. Wang and L. Q. Feng, Mater. Des., 2010, 31, 999 CrossRef CAS PubMed.
- Y. Liu, J. Liu, Z. W. Jiang and T. Tang, Polym. Degrad. Stab., 2012, 97, 214 CrossRef CAS PubMed.
- Y. Sato, Y. Kondob, K. Tsujitac and N. Kawai, Polym. Degrad. Stab., 2005, 89, 317 CrossRef CAS PubMed.
- W. R. Dang, M. Kubouchi and S. Yamamoto, IEEE Xplore, 2001, 980 Search PubMed.
- B. Bolasodun, A. Nesbitt, A. Wilkinson and R. Day, International Journal of Scientific & Technology Research, 2013, 2, 19 Search PubMed.
- G. Z. Jiang, S. J. Pickering, E. H. Lester and N. A. Warrior, Ind. Eng. Chem. Res., 2010, 49, 4535 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |