Preparation and characterization of novel positively charged copolymer composite membranes for nanofiltration
Abstract
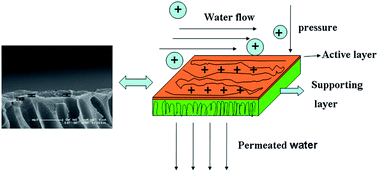
A novel copolymer poly(ether ether ketone) bearing pendant tertiary amine groups (TAPEEK-x) was synthesized by 3,3′-dimethylaminemethylene-4,4′-biphenol (DABP), 4,4′-bisfluorodiphenylketone (BFDPA) and bisphenol A (BPA). After reaction with iodomethane, the copolymer turned to poly(ether ether ketone) with pendant quaternary ammonium groups (QAPEEK-x). Afterward, QAPEEK-x copolymer was dissolved in formic acid and coated on PAN ultrafiltration membrane as a composite membrane. The NF membrane preparation conditions, such as drying temperature, formic acid concentration, copolymer concentration, feed concentration, and tolerance in chlorine solution were investigated. The experiments showed that the membrane whose coating polymer concentration was 0.5 wt% in 88% formic acid had the best flux of 12.6 L m−2 h−1 at 0.4 MPa and best rejection of 99.4% for 500 ppm MgCl2. In addition, the membrane showed excellent tolerance ability of chlorine with a little decrease of MgCl2 rejection. To some extent, this study provides a new insight to improve membrane separation capability and stability in the desalination process.

 Please wait while we load your content...
Please wait while we load your content...