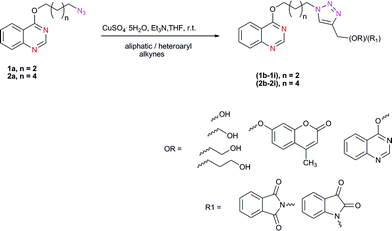
Synthesis of medicinally important quinazolines decorated with 1,4-disubstituted-1,2,3-triazoles using CuSO4·5H2O–Et3N catalytic system†
Monika Vashist,
Khushbu Kushwaha,
Reena Kaushik and
Subhash C. Jain*
Department of Chemistry, University of Delhi, Delhi, India. E-mail: jainsc48@hotmail.com; Fax: +91 11 27666605; Tel: +91 9810261947
First published on 29th April 2014
Abstract
The direct use of Cu(II) sulfate pentahydrate in the presence of triethylamine afforded 1,4-disubstituted-1,2,3-triazoles via 1,3-dipolar cycloaddition of terminal alkyne(s) to azide(s) at room temperature. A study on the additive effect of triethylamine in the presence of Cu(II) sulfate pentahydrate revealed that it is essential for the activation of the copper catalyst and is responsible for the reaction between aliphatic/aromatic heterocyclic alkyne(s) and azide(s), which otherwise did not react under the standard reaction conditions that are often used in click chemistry.
Introduction
Click chemistry has recently attracted considerable attention as a powerful and efficient way to obtain 1,2,3-triazoles in sufficient yields via a simple and benign procedure,1 and it is the fruit of a re-evaluation of previously reported azide alkyne chemistry.2–5 A copper catalyst in the form of Cu(I) and Cu(II) (e.g. CuI and CuSO4·5H2O) has been extensively used to regio-selectively6,7 obtain the 1,4-disubstituted triazole over its 1,5-regioisomer. This reaction proceeds via a multistep mechanism8 as compared to its original concerted nature involving azide–copper and alkyne–copper complexes.1,2,3-Triazoles have a wide range of industrial applications in agrochemicals, and they are also used as corrosion inhibitors, dyes and optical brighteners. As pharmaceuticals, triazole derivatives are fairly stable to metabolic degradation,9a–d and they are capable of participating in hydrogen bonding and dipole–dipole interactions and therefore provide potential advantages for target binding and cell permeability enhancement.9e–g Therefore, we investigated the synthesis of some novel quinazoline-based 1,2,3-triazole analogues, in which the quinazoline scaffold is employed as the basic nucleus because it shows various biological activities such as anti-microbial,10 anti-fungal,11 and anti-cancer;12 moreover, it can act as anti-neoplastic13 agent, a phosphodiesterase 7 inhibitor14 and a c-Src inhibitor.15 Furthermore, we have used a new methodology to construct the 1,2,3-triazole moiety on the quinazoline via a lipophilic linker such that both of these biodynamic scaffolds are present in a single molecular framework in the target molecule.
Results and discussion
Earlier, Cu(I) catalysts were either used directly or generated in situ from Cu(II) salts by using reducing agents such as sodium ascorbate.16 When sodium ascorbate cannot be used in the reaction, excess base, usually TEA or DIPEA, is used in the presence of a stoichiometric amount of copper(I) salts (CuI,17 Cu(CH3CN)4PF6,18 CuBr(PPh3)4 or CuIP(OEt)3 (ref. 19 and 20)) or in situ by oxidation of metal turnings of copper.We herein report the direct use of a Cu(II) catalyst, CuSO4·5H2O, as a sole catalyst without using any additional reducing/oxidizing agents in the presence of a triethylamine additive for the exclusive synthesis of 1,4-regioisomers of 1,2,3-triazoles in high yields. Note that the products obtained did not require any further purification steps except recrystallisation (Scheme 2).
In majority of the reported procedures, the activation of the terminal alkynes is achieved by the formation of Cu acetylide with Cu(I) salts as the main catalyst precursors. However, there are some precedents in the literature, in which Cu(II) salts also affect the activation of the terminal alkynes. For example, Reddy and co-workers21 reported that Cu(OAc)2 as well as a biopolymer-supported Cu(II) catalyst ALG22 (copper-alginates) catalyzed the synthesis of the 1,4-regioisomer of 1,2,3-triazoles. Similarly, Cu(II)-hydrotalcite23 (heterogeneous catalyst), iron oxide nanoparticle24 and Cu(OTf)2 have also been shown25 to catalyze the Huisgen [3 + 2] cycloaddition.
In order to synthesise the requisite triazole derivatives, initially a template reaction between 4-(6-azidohexyloxy)quinazoline (2a) (synthesized in Scheme 1) and 2-propyn-1-ol was tried under the standard reaction conditions to obtain a model compound 2b using CuSO4·5H2O–Na–ascorbate in H2O–tBuOH (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in the absence of a base. Unfortunately, this reaction did not proceed at all. Then, different solvents such as THF, THF
1) in the absence of a base. Unfortunately, this reaction did not proceed at all. Then, different solvents such as THF, THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (3
H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), DMF, DCM, CH3CN, H2O were used in the presence of a stoichiometric amount of CuSO4·5H2O along with an excess of sodium ascorbate (Table 1) at room temperature (Scheme 2).
1), DMF, DCM, CH3CN, H2O were used in the presence of a stoichiometric amount of CuSO4·5H2O along with an excess of sodium ascorbate (Table 1) at room temperature (Scheme 2).
| S. No. | Catalyst | Conditions | Solvent used | Time (h) | Yield (%) |
|---|---|---|---|---|---|
| a All reactions were performed with stoichiometric amount of CuSO4·5H2O–Na ascorbate and the reaction did not proceed.b Reaction was carried out with 10–20 mol% of catalyst and 1.2 mmol of Et3N but the reaction did not proceed to completion, and the product was isolated by chromatography.c Reaction was performed with 1.0 mmol of azide, 1 mmol of 2-propyn-1-ol, 1.2 mmol of Et3N, 5 ml of solvent and 15 mol% catalyst.d Reaction was carried out with CuSO4·5H2O and Et3N separately. | |||||
| 1 | CuSO4·5H2O–Na ascorbate | r.t./Δ | H2O–t-BuOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
48 | —a |
| 2 | CuSO4·5H2O–Na ascorbate | r.t./Δ | THF | 48 | —a |
| 3 | CuSO4·5H2O–Na ascorbate | r.t./Δ | THF–H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
24 | —a |
| 4 | CuSO4·5H2O–Na ascorbate | r.t./Δ | DMF | 24 | —a |
| 5 | CuSO4·5H2O–Na ascorbate | r.t./Δ | CH3CN | 24 | —a |
| 6 | CuI–Et3N | r.t./Δ | H2O–t-BuOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
16 | 52b |
| 7 | CuI–Et3N | r.t./Δ | DMF | 16 | 55b |
| 8 | CuI–Et3N | r.t./Δ | THF | 16 | 60b |
| 9 | CuSO4·5H2O–Et3N | r.t. | THF | 1/2 | 98c |
| 10 | CuSO4·5H2O | r.t./Δ | THF | 12 | —d |
| 11 | Et3N | r.t./Δ | THF | 12 | —d |
However, the reaction even then did not occur in any of these reaction conditions. The above mentioned reaction was also carried out in the presence of copper(I) salt (CuI) and excess of sodium ascorbate,26 but it again failed. The template reaction was not only carried out at room temperature but also under reflux conditions; however, in both of these cases, the reaction did not proceed (Table 1, entry 1–5). Finally, the reaction was carried out in the presence of CuI/Et3N, in different solvents, and the results obtained are summarized in Table 1. Note that the reaction was proceeding with CuI/Et3N at room temperature, but it did not go to completion; moreover, even when reflux conditions were employed, the reaction did not go to completion (Table 1, entry 6–8). THF can be illustrated as a suitable solvent as compared to other solvents such as H2O–t-BuOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), DMF, and THF for the reaction (entry 8). Finally, by optimizing the reaction conditions, a combination of CuSO4·5H2O (15 mol%) and Et3N (1.2 mmol) in THF at room temperature was found to be the most effective as it afforded 2b in 98% yield in 30 minutes (Table 1, entry 9). We also tried to carry out cycloaddition reaction of 2a with 2-propyn-1-ol in the presence of a catalytic amount of either CuSO4·5H2O (15 mol%) or triethylamine (1.2 mmol) separately at room temperature as well as under reflux conditions using the standardized reaction conditions; however, unfortunately, the desired product was not obtained. These results clearly demonstrate that the reaction did not proceed in the absence of either the amine or CuSO4·5H2O (Table 1, entry 10 and 11); hence, the presence of both is required for the reaction to occur.
1), DMF, and THF for the reaction (entry 8). Finally, by optimizing the reaction conditions, a combination of CuSO4·5H2O (15 mol%) and Et3N (1.2 mmol) in THF at room temperature was found to be the most effective as it afforded 2b in 98% yield in 30 minutes (Table 1, entry 9). We also tried to carry out cycloaddition reaction of 2a with 2-propyn-1-ol in the presence of a catalytic amount of either CuSO4·5H2O (15 mol%) or triethylamine (1.2 mmol) separately at room temperature as well as under reflux conditions using the standardized reaction conditions; however, unfortunately, the desired product was not obtained. These results clearly demonstrate that the reaction did not proceed in the absence of either the amine or CuSO4·5H2O (Table 1, entry 10 and 11); hence, the presence of both is required for the reaction to occur.
The scope and generality of the reaction in the presence of CuSO4·5H2O/Et3N were therefore investigated by carrying out the reaction between various aliphatic/aromatic heterocyclic alkynes and two different azides (1a and 2a) separately using the standard reaction conditions (Table 2). It was observed that the reaction time was less and yield was more in case of reaction between 4-(6-azidohexyloxy)quinazoline (2a) and alkynes as compared to that between 4-(4-azidobutyloxy)quinazoline (1a) and alkynes. Thus, it may be concluded that the azide with increased chain length is more stabilized under the present reaction conditions. The scope of the reaction was also examined with respect to various aliphatic alkynes. In contrast to results obtained in case of other azides (1a and 2a), the reaction was slow and resulted in decreased yields with increase in the chain length of aliphatic alkynes.
These results prompted us to further investigate the effect of various aromatic heterocyclic alkynes as compare to aliphatic alkynes. Results revealed that reaction was also proceeding with heterocyclic alkynes under the standardized reaction conditions (Table 1); moreover, the latter species reacted faster with the azides (1a and 2a) with comparatively more yield. Furthermore, a comparison between the O-alkylated and N-alkylated aromatic heterocyclic alkynes suggested a slow reaction occurs in the case of the O-alkylated alkyne without any significant effect on the yields (Table 2).
Conclusions
In conclusion, a combination of rather inexpensive copper sulfate pentahydrate and triethylamine not only resulted in the initiation of the 1,3-cycloaddition reaction but also yielded the requisite series of 1,4-regioisomers of novel 1,2,3-triazole analogues decorated with the quinazolin-3H-4-alkoxy moiety. The products were obtained in high yields using short reaction times. This combination of inexpensive copper sulfate pentahydrate and triethylamine was found to be an effective catalyst for the reaction between various azides and alkynes. The application of this methodology for the synthesis of various other hybrid targets is currently under investigation in our laboratory.Experimental section
All starting materials were of GR (Guaranteed Reagent) quality from Merck and all solvents used were of HPLC/AR grade. All spectroscopic measurements were performed at room temperature, 25 ± 1 °C. Melting points were determined using a Tropical Lab equip apparatus and were uncorrected. IR (KBr) spectra were recorded on a Perkin-Elmer FTIR spectrophotometer and the values are expressed as νmax cm−1. Mass spectral data were recorded on a Waters micromass LCT Mass Spectrometer. 1H NMR and 13C NMR spectra were recorded on a JEOL JNM ECX-400P at 400 MHz using TMS as an internal standard. The chemical shift values are recorded on a δ scale and the coupling constants (J) are in Hertz.General procedure for the synthesis of azides 1a and 2a
The respective starting bromo compounds 4-(4-bromobutyloxy)quinazoline (1 g, 3.55 mmol) (1) and 4-(6-bromohexyloxy)quinazoline (0.81 g, 2.64 mmol) (2) were separately stirred with sodium azide (1 × 1.5 times) in DMF (5 ml) under reflux condition (80 °C) overnight. After the completion of the reaction, the reaction mixture was poured over crushed ice and the contents were extracted with chloroform. The resulting organic layer was dried over sodium sulfate. Then, the solvent was evaporated under vacuum to obtain the required compounds as yellow coloured oils, which were further used without any additional purification. The respective precursors, i.e. bromo compounds 1 and 2, were synthesized by reacting quinazolin-3H-4-one27 with 1,4-dibromobutane and 1,6-dibromohexane, respectively, in acetone at room temperature. After the completion of reaction, the solution was filtered and the filtrate was concentrated under reduced pressure to obtain the crude product, which was then purified by silica gel column (petroleum ether–ethyl acetate as the eluent) to obtain the monobrominated products as the primary compounds.General procedure for the synthesis of compounds (1b–1i) and (2b–2i)
Alkynes (1 mmol) were stirred with CuSO4·5H2O (15 mol%) and Et3N (1.2 mmol) in THF at room temperature for 15 minutes. Then, the azide (1a or 2a) (1 mmol) dissolved in THF was added to reaction mixture and the progress of the reaction was observed on TLC. After the completion of the reaction (1/2–9 hours), the solvent was evaporated under reduced pressure and crushed ice was poured into the reaction mixture to precipitate the solid, which was then filtered using vacuum. In case the solid did not precipitate the reaction mixture was extracted with ethyl acetate. Then, the organic layer was dried over anhydrous sodium sulfate. After the removal of the solvent under reduced pressure, the residue obtained was washed with hot petroleum ether in order to eliminate aliphatic impurities. Then, the compounds were crystallized using chloroform/methanol or chloroform/acetone.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 4.41 (t, 2H, J = 6.6 Hz, –OCH2–), 3.98 (t, 2H, J = 6.9 Hz, >NCH2–), 2.36 (s, 3H, –CH3), 1.85–1.78 (m, 2H), 1.68–1.64 (m, 2H); 13C NMR (CDCl3, 100 MHz, δ): 160.9, 160.0, 154.5, 153.2, 147.8, 134.2, 127.1, 126.8, 125.9, 113.1, 112.4, 111.3, 101.4, 61.6, 48.9, 45.1, 26.8, 25.5, 18.0 (–CH3); TOF ES+ m/z: 457 (M+).
), 4.41 (t, 2H, J = 6.6 Hz, –OCH2–), 3.98 (t, 2H, J = 6.9 Hz, >NCH2–), 2.36 (s, 3H, –CH3), 1.85–1.78 (m, 2H), 1.68–1.64 (m, 2H); 13C NMR (CDCl3, 100 MHz, δ): 160.9, 160.0, 154.5, 153.2, 147.8, 134.2, 127.1, 126.8, 125.9, 113.1, 112.4, 111.3, 101.4, 61.6, 48.9, 45.1, 26.8, 25.5, 18.0 (–CH3); TOF ES+ m/z: 457 (M+).Acknowledgements
Monika Vashist and Khushbu Kushwaha are thankful to UGC and CSIR, New Delhi, respectively, for the research fellowship.Notes and references
- For reviews, see: (a) H. C. Kolb, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2001, 40, 2004 CrossRef CAS; (b) V. D. Bock, H. Hiemstra and J. H. van Maarseveen, Eur. J. Org. Chem., 2006, 51 CrossRef CAS; (c) M. V. Gil and M. J. Arévalo López, Synthesis, 2007, 1589 CrossRef CAS PubMed.
- (a) Almost 600 papers have been published since 2002, the year of the discovery of the copper (I) catalysis (personal communication by K. B. Sharpless); (b) G. Broggini, G. Molteni and G. Zecchi, Synthesis, 1995, 647 CrossRef CAS PubMed; (c) G. Broggini, G. Molteni, A. Terraneo and G. Zecchi, Tetrahedron, 1999, 55, 14803 CrossRef CAS; (d) I. Akritopoulou-Zanze, V. Gracias and S. W. Djuric, Tetrahedron Lett., 2004, 45, 8439 CrossRef CAS PubMed; (e) S. Chandrasekhar, C. L. Rao, C. Nagesh, C. R. Reddy and B. Sridhar, Tetrahedron Lett., 2007, 48, 5869 CrossRef CAS PubMed.
- (a) W. Lwowski, in 1,3-Dipolar Cycloaddition Chemistry., 1, ed. A. Padwa, Wiley, New York, 1984, p. 559 Search PubMed; (b) H. Wamhoff, Comprehensive Heterocyclic Chemistry., 5, ed. A. R. Katritzky and C. W. Rees, Pergamon Press, Oxford, 1984, p. 669 Search PubMed.
- R. B. Woodward and R. Hoffmann, Angew. Chem., 1969, 81, 797 (Angew. Chem., Int. Ed. Engl., 1969, 8, 781) CrossRef.
- (a) R. Huisgen, Angew. Chem., 1963, 75, 604 (Angew. Chem., Int. Ed. Engl., 1963, 2, 565) CrossRef CAS; (b) R. Huisgen, Angew. Chem., 1963, 75, 741 (Angew. Chem., Int. Ed. Engl., 1963, 2, 633) CrossRef.
- V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, Angew. Chem., 2002, 114, 2708 (Angew. Chem., Int. Ed., 2002, 41, 2596) CrossRef.
- C. W. Tornoe, C. Christensen and M. Meldal, J. Org. Chem., 2002, 67, 3057 CrossRef CAS PubMed.
- (a) V. O. Rodionov, V. V. Fokin and M. G. Finn, Angew. Chem., 2005, 117, 2250 (Angew. Chem., Int. Ed., 2005, 44, 2211) CrossRef; (b) V. D. Bock, H. Hiemstra and J. H. V. Maarseeven, Eur. J. Org. Chem., 2006, 51 CrossRef CAS.
- (a) W. Q. Fan and A. R. Katritzky, in Comprehensive Heterocyclic Chemistry II, ed. A. R. Katritzky, C. W. Rees and E. F. V. Scriven, Elsevier Science, Oxford, 1996, vol. 4, p. 1 Search PubMed; (b) B. S. Holla, M. Mahalinga, M. S. Karthikeyan, B. Poojary, P. M. Akberali and N. S. Kumari, Eur. J. Med. Chem., 2005, 40, 1173 CrossRef CAS PubMed; (c) M. A. Elmorsi and A. M. Hassanein, Corros. Sci., 1999, 41, 2337 CrossRef CAS; (d) D. K. Kim, J. Kim and H. J. Park, Bioorg. Med. Chem. Lett., 2004, 14, 2401 CAS; (e) D. K. Dalvie, A. S. Kalgutkar, S. C. Khojasteh-Bakht, R. S. Obach and J. P. O'Donnell, Chem. Res. Toxicol., 2002, 15, 269 CrossRef CAS; (f) W. S. Horne, M. K. Yadav, C. D. Stout and M. R. Ghadiri, J. Am. Chem. Soc., 2004, 126, 15366 CrossRef CAS PubMed; (g) S. G. Agalave, S. R. Maujan and V. S. Pore, Chem.–Asian J., 2011, 6, 2696 CrossRef CAS PubMed.
- S. G. Bubbly, S. B. Gudennavar, N. M. N. Gowda, R. Bhattacharjee, V. Gayathri and S. Natarajan, J. Chem. Crystallogr., 2012, 42, 305 CrossRef CAS.
- S. Tiwari, V. Mujaldai, V. Sharma, P. Saxwna and M. Shrivastava, Asian J. Pharm. Clin. Res., 2012, 5 Search PubMed.
- Y. Sheng-Jiao, L. Jun, D. Ying, P. Qiong, F. Yin-Xian and Z. Ji-Hong, RSC Adv., 2013, 3, 5563 Search PubMed.
- C. M. Cheng, Y. J. Lee, W. T. Wang, C. T. Hsu, J. S. Tsa, C. M. Wu, K. L. Ou and T. S. Yang, Biochem. Biophys. Res. Commun., 2011, 404(1), 297 CrossRef CAS PubMed.
- M. Redondo, J. G. Zarruk, P. Ceballos, D. I. Pérez, C. Pérez, A. P. Castillo, M. A. Moro, J. Brea, C. Val, M. I. Cadavid, M. I. Loza, N. E. Campillo, A. Martínez and C. Gil, Eur. J. Med. Chem., 2012, 47, 175 CrossRef CAS PubMed.
- F. Fei, L. Dong-Dong, Z. Hai-Liang, L. Jing-Ran, S. Jian, D. Qian-Ru and G. Hai-Bin, RSC Adv., 2013, 3, 26230 RSC.
- F. Himo, T. Lovell, R. Hilgraf, V. V. Rostovtsev, L. Noodleman, K. B. Sharpless and V. V. Fokin, J. Am. Chem. Soc., 2005, 127, 210 CrossRef CAS PubMed.
- C. W. Tornoe, C. Christensen and M. Meldal, J. Org. Chem., 2002, 67, 3057 CrossRef CAS PubMed.
- V. Aucagne and D. Leigh, Org. Lett., 2006, 8, 4505 CrossRef CAS PubMed.
- W. Peng, A. K. Feldman, A. K. Nugent, C. J. Hawker, A. Scheel, B. Voit, J. Pyun, J. M. J. Fréchet, K. B. Sharpless and V. V. Fokin, Angew. Chem., 2004, 43, 3928 Search PubMed.
- M. Malkoch, K. Schleicher, E. Drockenmuller, C. J. Hawker, T. P. Russell, P. Wu and V. V. Fokin, Macromolecules, 2005, 38(9), 3663 CrossRef CAS.
- K. R. Reddy, K. Rajgopal and M. L. Kantam, Synlett, 2006, 6, 957 Search PubMed.
- K. R. Reddy, K. Rajgopal and M. L. Kantam, Catal. Lett., 2007, 114, 36 CrossRef CAS.
- S. Fukuzawa, E. Shimizu and S. Kikuchi, Synlett, 2007, 15, 2436 CrossRef PubMed.
- K. Ahmed and S. Ponnampalli, RSC Adv., 2013, 3, 7419 RSC.
- K. Namitharan, M. Kumarraja and K. Pitchumani, Chem.–Eur. J., 2009, 15, 2755 CrossRef CAS PubMed.
- K. K. Ghosh, H. H. Ha, N. Y. Kang, Y. Chandran and Y. T. Chang, Chem. Commun., 2011, 47, 7488 RSC.
- F. R. Alexandre, A. Berecibara and T. Bessonb, Tetrahedron Lett., 2002, 43, 3911 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra02123a |
| This journal is © The Royal Society of Chemistry 2014 |