Sensitive sandwich electrochemical immunosensor for human chorionic gonadotropin using nanoporous Pd as a label
Abstract
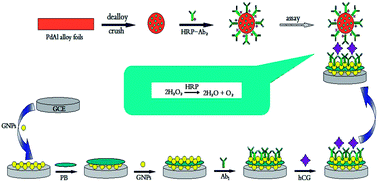
A novel sandwich-type electrochemical immunosensor for sensitive detection of human chorionic gonadotropin (hCG) was designed. The nanoporous Pd (NP-Pd) was prepared by a dealloying method and used as an electrochemical label due to its wonderful conductivity, good biocompatibility and strong electrocatalytic activity toward antigen–antibody reaction. Results proved that the immunosensor fabricated using the label based on NP-Pd loaded with horseradish peroxidase (HRP) and secondary anti-hCG antibody (Ab2) (NP-Pd–HRP–Ab2) had high sensitivity, and the sensitivity of the label NP-Pd–HRP–Ab2 was much higher than that of HRP–Ab2. Gold nanoparticles (GNPs), Prussian blue (PB) and GNPs as immobilization matrix were not only used to immobilize anti-hCG (Ab1) but also took part in the signal amplification. Under optimized conditions, the amperometric signal increased linearly with hCG concentration in the range of 0.5 ng mL−1 to 200 ng mL−1 (γ = 0.9986) with a low detection limit (3σ) of 9.2 pg mL−1 (0.093 mIU mL−1). The immunosensor displayed good sensitivity and selectivity. In addition, the immunosensor was successfully used for the determination of hCG in human serum.

 Please wait while we load your content...
Please wait while we load your content...