Lauraceae alkaloids†
Abstract
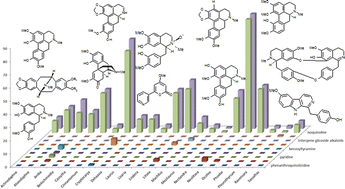
Lauraceae is one of the most representative botanical families, presenting 67 genera, with over 2500 species and more than 300 different alkaloids reported, mainly isoquinolines. Its diversity and relevance to chemosystematic relationships is presented and discussed.

 Please wait while we load your content...
Please wait while we load your content...